Abstract
We investigated the capacity of intrathecal arachidonyl-2’-chloroethylamide (ACEA), a cannabinoid-1 receptor (CB1R) agonist, to inhibit referred hyperalgesia and increased bladder contractility resulting from acute acrolein-induced cystitis in rats. 24 female rats were divided into 4 groups: 1) intrathecal vehicle/intravesical saline; 2) intrathecal vehicle/intravesical acrolein; 3) intrathecal ACEA/intravesical saline; and 4) intrathecal ACEA/intravesical acrolein. Bladder catheters were placed 4-6 days prior to the experiment. On the day of the experiment, rats were briefly anesthetized with isoflurane to recover the external end of the cystostomy catheter. After recovery from anesthesia, pre-treatment cystometry was performed, and mechanical sensitivity of the hindpaws was determined. Rats were again briefly anesthetized with isoflurane to inject ACEA or vehicle into the intrathecal space between L5-L6. Beginning 10 minutes after intrathecal injection, saline or acrolein was infused into the bladder for 30 minutes. Post-treatment cystometry and mechanical sensitivity testing were performed. Rats were euthanized, and bladders were collected, weighed, and fixed for histology. The intrathecal vehicle/intravesical acrolein group developed mechanical hyperalgesia with post-treatment mechanical sensitivity of 6 ± 0.3 g compared to pretreatment of 14 ± 0.4 g (p < 0.01). Pre- and post-treatment hind paw mechanical sensitivity was statistically similar in rats that received intrathecal ACEA prior to intravesical infusion of acrolein (15 ± 0.2 g and 14 ± 0.4 g, respectively). Acrolein treatment increased basal bladder pressure and maximal voiding pressure and decreased intercontraction interval and voided volume. However, intrathecal ACEA was ineffective in improving acrolein-related urodynamic changes. In addition, bladder histology demonstrated submucosal and muscularis edema that was similar for all acrolein-treated groups, irrespective of ACEA treatment. Intravesical saline had no effect on results of cystometry or mechanical sensitivity of the hind paws, regardless of intrathecal treatment. Intrathecal ACEA prevented referred hyperalgesia associated with acute acrolein–induced cystitis. However, in this experimental model, ACEA did not ameliorate the associated urodynamic changes. These findings suggest that pain arising from cystitis may be inhibited by activation of spinal CB1R but the acute local response of the bladder appeared to be unaffected by stimulation of spinal CB1R.
Keywords: CB1 receptor, rat, cystitis, hyperalgesia
Introduction
Bladder disorders are frequently characterized by pain and increased contractility. It has long been recognized that cannabinoids have the capacity to provide analgesia, and recent studies indicate that cannabinoids have the capacity to inhibit bladder contractility in vivo [1] and in vitro [2], as well as suppressing noxious afferent input from the bladder [3]. However, the majority of in vivo studies have entailed systemic administration of cannabinoids or inhibitors of fatty acid amide hydrolase, the enzyme primarily responsible for degradation of the endocannabinoid anandamide (AEA) [4]. Anandamide has the capacity to bind to the primary cannabinoid receptors CB1R and CB2R, as well as the transient receptor vanilloid potential 1 (TRPV1) and GPR55 [4,5]. Receptors for cannabinoids have been identified in the spinal cord, dorsal root ganglia, and bladder. Despite the use of systemic CB1R and CB2R antagonists, as well as studies performed with mice deficient for functional CB1R [6], it is unclear whether the effects of cannabinoids on bladder pain and contractility are due to activation of receptors within the bladder, peripheral nerves, and/or central nervous system.
The present study was performed to specifically investigate participation of spinal CB1R in regulation of bladder contractility and sensitization of peripheral somatic afferent pathways in the presence of bladder inflammation. It was recently reported that intrathecal instillation of a FAAH inhibitor suppressed increased bladder contractility due to partial urethral obstruction with or without intravesical instillation of prostaglandin E2 [7]. However, increased anandamide within the intrathecal space could have exerted its effects via any of the receptors to which it binds. The specific capacity of spinal CB1R receptors to modulate responses to bladder inflammation remains unclear. These experiments were designed to specifically evaluate the capacity of intrathecal arachidonyl-2’-chloroethylamide (ACEA), a selective CB1R agonist [8], to attenuate referred hyperalgesia and increased bladder contractility associated with acrolein-induced cystitis. In the model of acrolein-induced cystitis, rats not only developed changes in urodynamics but also exhibit referred somatic hyperalgesia similar to that observed in Interstitial Cystitis/Painful Bladder (IC/PBS) patients [9].
Materials and methods
Animals
Adult female Wistar rats (225-310 g) were used in these experiments and housed in standard care facilities with water and food ad libitum on a 12 hour light-dark cycle. All animal protocols were reviewed and approved by the Animal Care and Use Committee of the University of Wisconsin. There were 4 groups of rats treated as outlined in Table 1 with each group including 6 rats.
Table 1.
Experimental groups
| Group | Intrathecal | Intravesical |
|---|---|---|
| Veh/sal | vehicle | 0.9% saline |
| Veh/acro | vehicle | 1 mM acrolein |
| ACEA/sal | 100 μg ACEA | 0.9 % saline |
| ACEA/acro | 100 μg ACEA | 1 mM acrolein |
Each group included 6 adult female Wistar rats. 1 mM acrolein (acro) was dissolved in normal saline. 100 μg ACEA was dissolved in the vehicle which was v:v:v, 2% Tween-80: 15% dimethyl sulfoxide: 83% normal saline.
Bladder catheterization
Bladder catheters were surgically implanted in rats 4-6 days before experiments. Rats were treated perioperatively for 96 hours with trimethoprim-sulfmethoxazole (40 mg-200 mg/250 mL, final concentration) in their drinking water. On day of catheter implantation, rats were anesthetized with isoflurane (5% for induction and 2% for maintenance), and 15 μg of buprenorphine in 1 mL of normal saline was given subcutaneously for postoperative analgesia. The dorsal neck and lower abdomen were clipped, prepped with 1% povidone iodine, and sterilely draped. A 4 cm incision was made in the lower abdomen to expose the bladder, and a small incision was made in the dome. A 25 cm polyethylene-50 catheter with a cuff was inserted into the bladder and secured with a 6-0 silk purse-string suture. The catheter was tunneled subcutaneously to the dorsal neck, anchored, and the free end was stoppered with a blunted 22 gauge needle. The skin was closed with three 4-0 silk interrupted sutures, and the abdominal wound was closed with 4-0 silk suture. The catheter was covered with a dressing and secured to the rat’s back. Animals were allowed to recover and then returned to the animal care facility where they were housed individually until the day of experiment.
Cystometry and mechanical sensitivity
On the day of experimentation, individual rats were briefly anesthetized with isoflurane (5% induction and 2% maintenance), weighed, and the catheter dressing removed. Rats were placed in a Plexiglas chamber (25 × 8 × 9 cm) with a wire mesh floor. The bladder catheter was connected to a three-way stopcock connected to an infusion pump and pressure transducer. Rats were allowed to recover from anesthesia and acclimate to the chamber for 30-40 minutes.
Baseline mechanical sensitivity of both hindpaws was determined using the up-down threshold technique with von Frey monofilaments (2, 4, 6, 8, and 15 g) [10]. Mechanical sensitivity of the hindpaws has been evaluated as indicative of referred hyperalgesia from visceral inflammation [11]. Starting at 2 g, filaments were applied perpendicularly to the plantar surface of the paw until there was bending of the filament. A withdrawal of the paw or licking was considered a positive response. If there was no response, the next filament in the sequence was tested. If a positive response was elicited, the next weaker filament was tested. Once the weakest filament causing a positive response was identified, the testing series was repeated. Four to six testing series were performed per animal.
Completion of testing of baseline mechanical sensitivity was followed by performance of pre-treatment cystometry. Normal saline was infused into the bladder at 10 mL/hour for 1 hour. Urodynamic measurements during the final 40 minutes were utilized for data analysis. Also during this time, voided saline was collected on pre-weighed labliner paper. The volume of saline voided per micturition event was determined from the mass of voided saline and its density (1.046 g/mL).
The animal was again briefly anesthetized (10-15 minutes) with isoflurane, and an intrathecal injection (20 μL) of vehicle (v:v:v, 2% Tween-80: 15% dimethyl sulfoxide: 83% normal saline) or 100 μg ACEA in vehicle was administered via the L5-L6 interspace with a 1.5 inch 27 gauge needle attached to a 25 μL Hamilton glass syringe. The rat was returned to the chamber, and the bladder catheter was reconnected to the stopcock. Ten minutes after intrathecal injection, 1 mM acrolein (in saline) or normal saline was infused intravesically for 30 minutes at a rate of 2 mL/hour. Post-treatment cystometry was performed at the conclusion of infusion of acrolein or saline by infusing saline infusion at 10 mL/hour for 1 hour as described for pre-treatment cystometry. Mechanical sensitivity of the hindpaws was again determined as previously described after completion of cystometry. Rats were euthanized with 0.4 mL of intra-peritoneal Beuthanasia® (390 mg/mL pentobarbital and 50 mg/mL phenytoin) and perfused with 200 mL of 4°C normal saline via the left ventricle. Bladders were collected, and bladder weights were recorded.
Histology
Bladders were fixed in 2% paraformaldehyde overnight followed by cryoprotection with 30% sucrose in 0.1 M phosphate buffer overnight. Bladders were then frozen in Tissue-tek® O.T.C. compound, cryosectioned in 20 μm transverse sections, and sections were mounted on slides. Tissue sections were then stained with hematoxylin and eosin (H & E) and viewed with light microscopy.
Statistics
Data were compared using one-way ANOVA with Bonferroni post-hoc analysis. A p < 0.05 was considered significant.
Results
Urodynamics
Figure 1A is a representative pre-treatment cystometrogram. Pre-treatment urodynamic measurements were similar among all groups (Table 2). The post-treatment cystometrogram (Figure 1B) and urodynamic values of the veh/sal group were not statistically different when compared to the pre-treatment values (Table 2). Figure 1C is a representative post-treatment cystometrogram of a veh/acro-treated rat. When compared to pre-treatment values, basal bladder pressure (BBP) and maximal voiding pressure (MVP) were significantly increased, and intercontraction interval (ICI) and average voided volume (VV) per micturition were decreased (p < 0.05 for all comparisons) (Table 2). Figure 1D is a post-treatment cystometrogram of ACEA/sal. The pre- and post- treatment urodynamic measurements of this group were statistically the same (Table 2). Figure 1E is a representative post-treatment cystometrogram for ACEA/acro. Similar to the veh/acro group, BBP and MVP were significantly increased, and ICI and VV were significantly decreased (p < 0.05) when compared to pre-treatment (Table 2).
Figure 1.
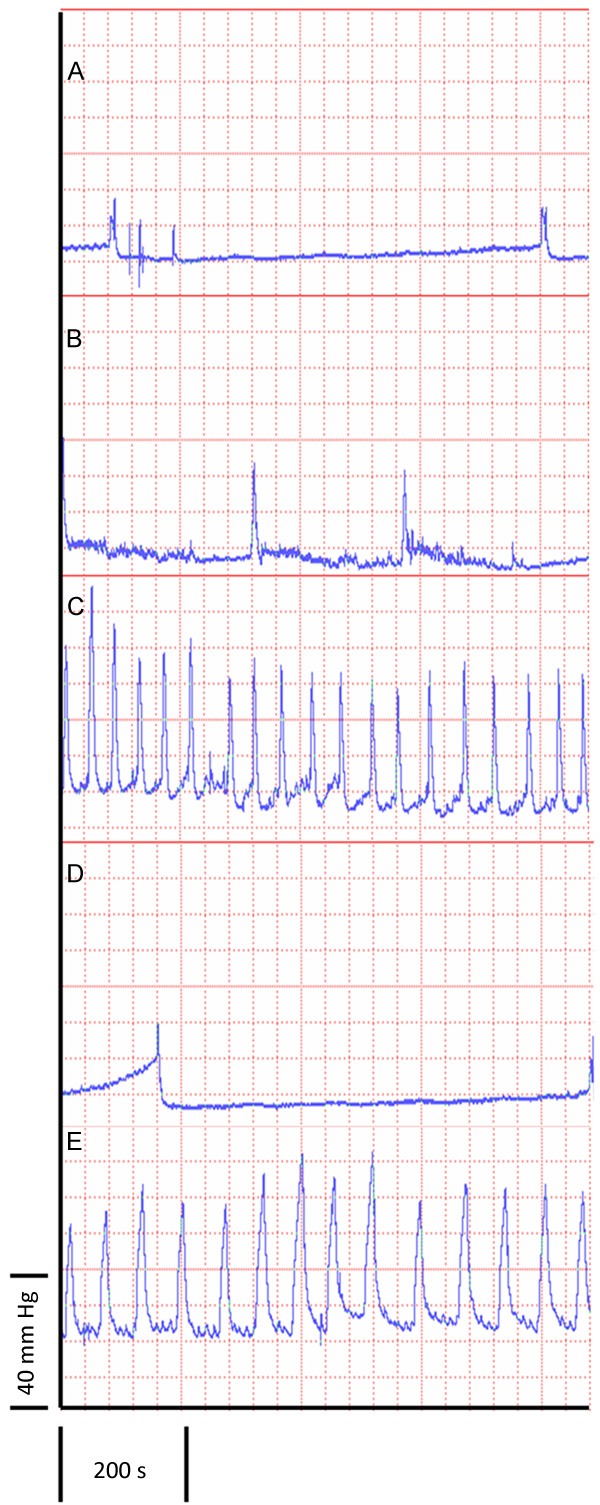
Representative cystometrograms. A. Representative pre-treatment cystometrogram. All pre-treatment cystometrograms were statistically similar (see Table 2). B-E. Representative post-treatment cystometrograms: B. veh/sal; C. veh/acro; D. ACEA/sal; and E. ACEA/acro.
Table 2.
Results of Cystometry
| veh/sal | veh/acro | ACEA/sal | ACEA/acro | ||
|---|---|---|---|---|---|
| Basal Bladder Pressure (mm Hg) (BBP) | Pre | 5.61 ± 0.379 | 5.08 ± 0.316 | 6.18 ± 0.239 | 4.39 ± 0.196 |
| Post | 4.74 ± 0.217 | 17.2 ± 0.387* | 5.64 ± 0.323 | 12.5 ± 0.323* | |
| Maximum Voiding Press (mm Hg) (MVP) | Pre | 38.5 ± 0.904 | 32.1 ± 0.786 | 32.0 ± 1.01 | 26.3 ± 0.838 |
| Post | 35.8 ± 0.766 | 59.1 ± 1.22* | 28.6 ± 0.654 | 54.2 ± 1.38* | |
| Intercontraction interval (s) (ICI) | Pre | 299 ± 5.50 | 248 ± 4.81 | 388 ± 14.6 | 352 ± 18.5 |
| Post | 286 ± 11.3 | 71 ± 3.16* | 468 ± 14.6 | 111 ± 5.49* | |
| Voided volume (μL) (VV) | Pre | 738 ± 26.7 | 742 ± 16.0 | 1080 ± 25.4 | 899 ± 52.5 |
| Post | 786 ± 38.0 | 190 ± 11.3* | 1360 ± 33.0 | 254 ± 12.3* |
Values reported as mean ± SEM (standard error of the mean). Pre = pre-treatment. Post = post-treatment.
indicates significant difference (p < 0.05) compared to pre-treatment.
Mechanical sensitivity of hindpaws
Pre-treatment mechanical hindpaw sensitivity was statistically similar between all groups, 14 ± 0.4 g (mean ± standard error of the mean (SEM)) for veh/sal, 14 ± 0.4 g for veh/acro, 15 ± 0.3 g for ACEA/sal, and 15 ± 0.2 g for ACEA/acro (Figure 2). Post-treatment mechanical sensitivity was statistically the same as the pre-treatment values for veh/sal (13 ± 0.5 g), ACEA/sal (13 ± 0.5 g), and ACEA/acro (14 ± 0.4 g). The veh/acro group developed mechanical hyperalgesia as demonstrated by a statistically significant increase in mechanical sensitivity (6 ± 0.3 g post-treatment) compared to pretreatment (14 ± 0.4 g; p < 0.01). In addition, the post-treatment mechanical sensitivity of the veh/acro group (6 ± 0.3 g) was statistically increased as compared to post-treatment mechanical sensitivity of the ACEA/acro (14 ± 0.4 g) (p < 0.01). These data demonstrate that intrathecal administration of ACEA prevented development of referred hyperalgesia.
Figure 2.
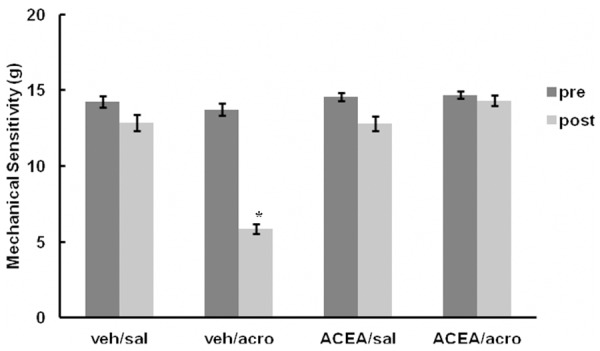
Mechanical sensitivity of hindpaws. Mechanical sensitivity of hindpaws. Mechanical sensitivity studies were conducted using von Frey monofilaments. Pre = pre-treatment. post = post-treatment. Error bars represent standard error of the mean (SEM). Veh/acro post-treatment mechanical sensitivity was statistically increased compared to the pre-treatment value indicating development of hyperalgesia (*p < 0.01). It was also statistically greater than post-treatment ACEA/acro, indicating that ACEA prevented development of hyperalgesia (*p < 0.01).
Bladder weight and histology
Bladder masses were statistically greater in rats that received intravesical acrolein (veh/acro, 0.359 ± 0.034 g, and ACEA/acro, 0.441 ± 0.032 g), irrespective of intrathecal ACEA (p < 0.01), compared to those that received intravesical saline (veh/sal, 0.198 ± 0.022 g and ACEA/sal, 0.230 ± 0.013 g) (Figure 3A). Figure 3B-E are H & E stained bladder sections representative of the 4 groups. Histological evaluation of the bladders consistently showed increased edema in the submucosa and muscularis layers of the groups receiving intravesical acrolein (Figure 3C and 3E), while normal bladder architecture was observed in animals receiving intravesical saline (Figure 3B and 3D).
Figure 3.
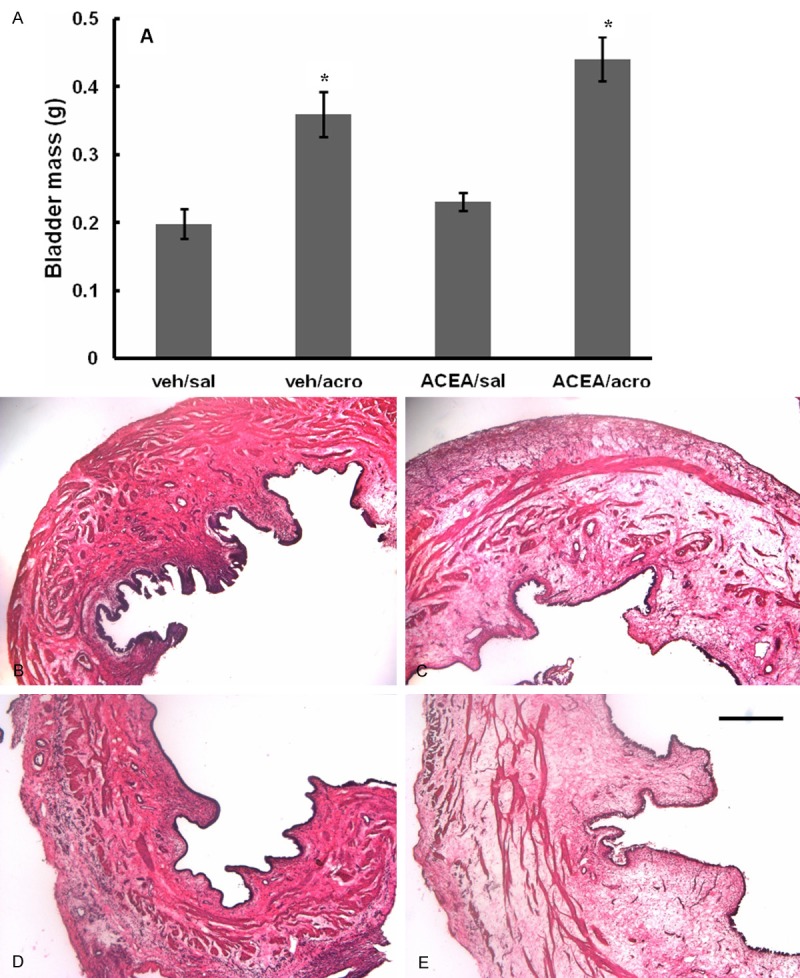
Mean weights and histological sections of bladders. A. Bladder mass increased after treatment with acrolein, irrespective of ACEA treatment (*p < 0.05). Error bars represent SEM. B-E. Hematoxylin-eosin stained histological sections of bladders post-treatment: B. veh/sal; C. veh/acro; D. ACEA/sal; and E. ACEA/acro. Treatment with acrolein resulted in edema in the submucosa and muscularis, irrespective of ACEA, while treatment with saline did not induce edema. The scale bar in E is 50 μm.
Discussion
A consistent cannabinoid therapeutic goal is improved efficacy of treatment with minimal undesirable side effects. Identification of which cannabinoid receptors regulate various physiological processes and the location of the relevant receptor(s) responsible for these effects should improve outcomes of treatment utilizing cannabinoids while minimizing side effects. Systemic administration of cannabinoids has been demonstrated to provide analgesia in experimental models of inflammatory pain [12,13]. However, this method of delivery cannot delineate the specific and selective effects of activation of the CB1R, CB2R or other cannabinoid receptors within the spinal cord, peripheral nerves, or bladder on afferent sensitization or bladder contractility. Similarly, observation of the presence of cannabinoid receptors within various tissues alone does not confirm activity of these receptors when exposed to cannabinoids. Therefore, our study was designed to determine the specific effects of intrathecal ACEA, a selective CB1R agonist [8], on referred hyperalgesia and increased bladder contractility associated with acute acrolein-induced cystitis. Intrathecal CB1R agonist is an alternative route of administration to systemic that has been demonstrated to be antihyperalgesic and antiallodynic in models of somatic pain [14,15]. However, this has not been investigated in visceral pain. The acrolein-induced cystitis model of visceral pain results in bladder wall edema and hemorrhagic cystitis, a feature seen in some patients treated with cyclophosphamide [9].
In the current study, intravesical acrolein caused urodynamic changes and increased peripheral mechanical sensitivity similar to that observed in prior studies [11,16]. Intrathecal administration of ACEA, inhibited referred peripheral mechanical hyperalgesia, but had no effect on increased bladder contractility associated with acrolein-induced cystitis. This observation suggests that signaling via spinal CB1 receptors has little effect on bladder contractility induced by acute inflammation. The mechanisms by which intrathecal CB1R agonists reduce hyperalgesia have not been clearly elucidated. CB1R are located both on presynaptic afferents and postsynaptic interneurons at the level of the spinal cord [17]. Prior studies comparing normal and CB1R (on peripheral nerves) knockout mice suggest that not only are there peripheral anti-hyperalgesia mechanisms but also spinal postsynaptic mechanisms. In the absence of peripheral CB1R on afferents, hyperalgesia was inhibited by intrathecal administration of a CB1R agonist [18]. In addition, using similar CB1R knockout mice, Kato et al. demonstrated that CB1R on peripheral nerves were necessary for long term depression of excitatory synapses in primary afferents [19]. Moreover, a CB1R agonist decreased C-fiber post-discharge response in repetitive stimulation that is indicative of hyperexcitability [20].
Administration of ACEA 1.5 hours before mechanical sensitivity tests were conducted prevented development of referred mechanical hyperalgesia in our study. These results indicate that 1) the actions of intrathecal ACEA last longer than one hour and/or 2) the effects of the stimulus (e.g., intravesical acrolein) on referred mechanical hyperalgesia could be modified by prior administration of a CB1R agonist. Prior studies from our laboratory have demonstrated that intrathecal lidocaine inhibits referred mechanical hyperalgesia when administered prior to intravesicular acrolein infusion but was ineffective when administered after instillation of acrolein, even though this local anesthetic is often used to treat pain after the inciting event [11]. While we did not investigate the effects of intrathecal ACEA given after hyperalgesia develops, it is likely that it would also be effective as demonstrated by previous studies of chronic somatic pain where in which CB1R agonists abated hyperalgesia [14,21].
Intrathecal administration of ACEA had no significant effect on urodynamic changes caused by intravesical acrolein. This is particularly interesting in light of the report that intrathecal administration of an inhibitor of AEA degradation prevented urodynamic changes induced by partial bladder outlet obstruction or prostaglandin E2 [7]. As mentioned previously, differential findings between that study and the current report may be due to differences in experimental design or activation CB2R (or other signaling pathways) by increased spinal AEA.
Bladder edema, as evidenced by increased bladder weight and thickening of the submucosa and muscularis, caused by acrolein was unchanged by administration of intrathecal ACEA. We previously reported that intrathecal lidocaine had no effect on histological changes induced in the bladder by acrolein [11]. This was also observed in studies of arthritis in which joint inflammation was unchanged by intrathecal administration of cannabinoids [22]. These combined results suggest that spinal signaling has little or no effect on local tissue response to inflammatory stimuli.
In conclusion, the results of this study demonstrate that intrathecal ACEA prevents referred hyperalgesia at the spinal level in an acute model of acrolein-induced cystitis in rats without changes in urodynamics or bladder inflammation. Our findings suggest that intrathecal CB1R agonists may be an effective treatment for pain associated with cystitis.
Acknowledgements
This research was supported by NIH R01 DK088806 (DEB) and a Foundation for Anesthesia Education and Research (FAER) Graduate Research Fellowship (MRJ).
Disclosure of conflict of interest
None.
References
- 1.Gandaglia G, Strittmatter F, La Croce G, Benigni F, Bettiga A, Castiglione F, Moschini M, Mistretta F, Gratzke C, Montorsi F, Stief C, Hedlund P. The fatty acid amide hydrolase inhibitor oleoyl ethyl amide counteracts bladder overactivity in female rats. Neurourol Urodyn. 2014;33:1251–1258. doi: 10.1002/nau.22482. [DOI] [PubMed] [Google Scholar]
- 2.Tambaro S, Casu MA, Mastinu A, Lazzari P. Evaluation of selective cannabinoid CB(1) and CB(2) receptor agonists in a mouse model of lipopolysaccharide-induced interstitial cystitis. Eur J Pharmacol. 2014;729:67–74. doi: 10.1016/j.ejphar.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Aizawa N, Hedlund P, Füllhase C, Ito H, Homma Y, Igawa Y. Inhibition of peripheral fatty acid amide hydrolase depresses activities of bladder mechanosensitive nerve fibers of the rat. J Urol. 2014;192:956–963. doi: 10.1016/j.juro.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Cravatt B, Lichtman A. The endogenous cannabinoid system and its role in nociceptive behavior. J Neurobiol. 2004;61:149–160. doi: 10.1002/neu.20080. [DOI] [PubMed] [Google Scholar]
- 5.Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Füllhase C, Campeau L, Sibaev A, Storr M, Hennenberg M, Gratzke C, Stief C, Hedlund P, Andersson KE. Bladder function in a cannabinoid receptor type 1 knockout mouse. BJU Int. 2014;113:144–151. doi: 10.1111/bju.12350. [DOI] [PubMed] [Google Scholar]
- 7.Fullhase C, Russo A, Castiglione F, Benigni F, Campeau L, Montorsi F, Gratzke C, Bettiga A, Stief C, Andersson KE, Hedlund P. Spinal cord FAAH in normal micturition control and bladder overactivity in awake rats. J Urol. 2013;189:2364–2370. doi: 10.1016/j.juro.2012.11.165. [DOI] [PubMed] [Google Scholar]
- 8.Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- 9.Rubin J, Rubin R. Cyclophosphamide hemorrhagic cystitis. J Urol. 1966;96:313–316. doi: 10.1016/S0022-5347(17)63260-9. [DOI] [PubMed] [Google Scholar]
- 10.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 11.Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Lidocaine prevents referred hyperalgesia associated with cystitis. Neurourol Urodyn. 2009;28:455–460. doi: 10.1002/nau.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyson A, Peacock M, Chen A, Courade J, Yaqoob M, Groarke A, Brain C, Loong Y, Fox A. Antihyperalgesic properties of the cannabinoid CT-3 in chronic neuropathic and inflammatory pain states in the rat. Pain. 2005;116:129–137. doi: 10.1016/j.pain.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Farquhar-Smith WP, Rice AS. Administration of endocannabinoids prevents a referred hyperalgesia associated with inflammation of the urinary bladder. Anesthesiology. 2001;94:507–513. doi: 10.1097/00000542-200103000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Martin WJ, Loo CM, Basbaum AI. Spinal cannabinoids are anti-allodynic in rats with persistent inflammation. Pain. 1999;82:199–205. doi: 10.1016/S0304-3959(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 15.Horvath G, Kekesi G, Nagy E, Benedek G. The role of TRPV1 receptors in the antinociceptive effect of anandamide at spinal level. Pain. 2008;134:277–284. doi: 10.1016/j.pain.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Meotti F, Forner S, Lima-Garcia J, Viana A, Calixto J. Antagonism of the transient receptor potential ankyrin 1 (TRPA1) attenuates hyperalgesia and urinary bladder overactivity in cyclophosphamide-induced haemorrhagic cystitis. Chem Biol Interact. 2013;203:440–447. doi: 10.1016/j.cbi.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Salio C, Fischer J, Franzoni MF, Conrath M. Pre- and postsynaptic localizations of the CB1 cannabinoid receptor in the dorsal horn of the rat spinal cord. Neuroscience. 2002;110:755–764. doi: 10.1016/s0306-4522(01)00584-x. [DOI] [PubMed] [Google Scholar]
- 18.Dableh LJ, Yashpal K, Henry JL. Physiological evidence of a postsynaptic inhibition of the tail flick reflex by a cannabinoid receptor agonist. Eur J Pharmacol. 2009;602:36–40. doi: 10.1016/j.ejphar.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato A, Punnakkal P, Pernía-Andrade AJ, von Schoultz C, Sharopov S, Nyilas R, Katona I, Zeilhofer HU. Endocannabinoid-dependent plasticity at spinal nociceptor synapses. J Physiol. 2012;590:4717–4733. doi: 10.1113/jphysiol.2012.234229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drew LJ, Harris J, Millns PJ, Kendall DA, Chapman V. Activation of spinal cannabinoid 1 receptors inhibits C-fibre driven hyperexcitable neuronal responses and increases [35S] GTPgammaS binding in the dorsal horn of the spinal cord of noninflamed and inflamed rats. Eur J Neurosci. 2000;12:2079–2086. doi: 10.1046/j.1460-9568.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 21.Starowicz K, Makuch W, Osikowicz M, Piscitelli F, Petrosino S, Di Marzo V, Przewlocka B. Spinal anandamide produces analgesia in neuropathic rats: possible CB(1)- and TRPV1-mediated mechanisms. Neuropharmacology. 2012;62:1746–1755. doi: 10.1016/j.neuropharm.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Petrovszki Z, Kovacs G, Tomboly C, Benedek G, Horvath G. The Effects of Peptide and Lipid Endocannabinoids on Arthritic Pain at the Spinal Level. Anesth Analg. 2012;114:1346–1352. doi: 10.1213/ANE.0b013e31824c4eeb. [DOI] [PubMed] [Google Scholar]


