Figure 2.
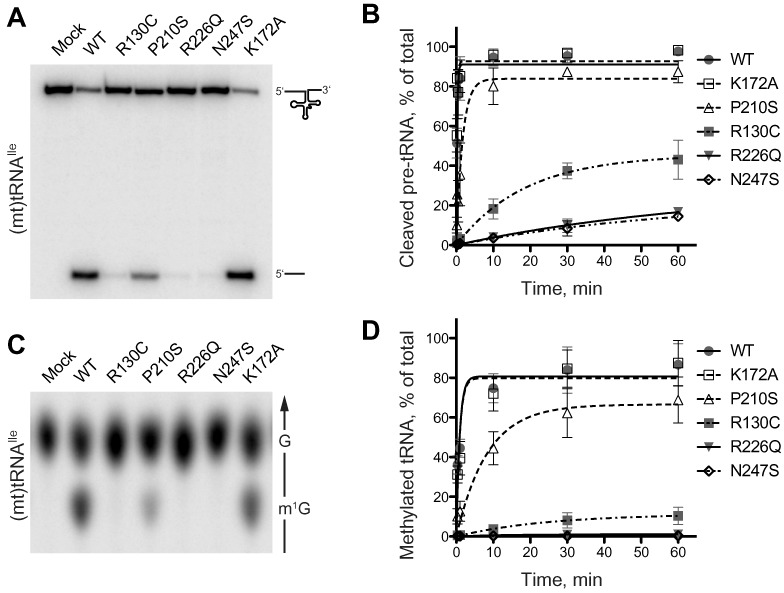
Mutations in SDR5C1 affect the tRNA-maturation activities of the mtRNase P complex. (A and B) mtRNase P was reconstituted from recombinant PRORP, TRMT10C, and wild-type or mutant SDR5C1, and its activity assayed with a 5′ labeled (mt)tRNAIle precursor. Reaction aliquots were withdrawn and stopped at different time points and resolved by denaturing PAGE. In (A), the 1-minute time point of a representative experiment is shown. (B) Cleavage data of six complete, independent experiments were plotted as means and SD, and fit by nonlinear regression. (C and D) The methyltransferase subcomplex of mtRNase P was reconstituted from recombinant TRMT10C and wild-type or mutant SDR5C1, and its activity assayed with position-9 labeled (mt)tRNAIle and SAM. Reaction aliquots were withdrawn and stopped at different time points and the tRNA hydrolysate resolved by thin-layer chromatography (TLC). In (C), the 1-minute time point of a representative experiment is shown. The direction of migration and the positions of G and m1G are indicated to the right; only the informative part of the TLC is shown. (D) Methylation data of five complete, independent experiments were plotted as means and SD, and fit by nonlinear regression.
