Figure 3.
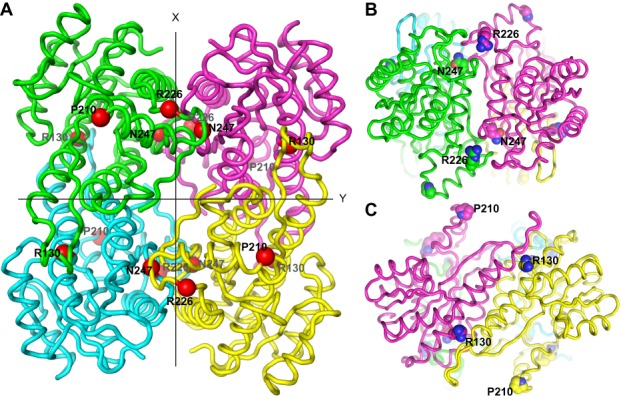
SDR5C1 structure and location of the studied pathogenic mutations. (A) Ribbon-display of the crystal structure of SDR5C1 in tetrameric form (PDB: 1U7T), as viewed along one of three mutually perpendicular dyad axes. The subunits interfaces are arranged about the two other axes, labeled X and Y. The α-carbons of the amino acids affected by the studied mutations are highlighted as red spheres and labeled. (B) SDR5C1 tetramer viewed along the X-axis indicated in (A). The side chains of the amino acids affected by the studied mutations are highlighted by ball-display and labeled. (C) SDR5C1 tetramer viewed along the Y-axis indicated in (A). The side chains of the amino acids affected by the studied mutations are highlighted by ball-display and labeled.
