Figure 8.
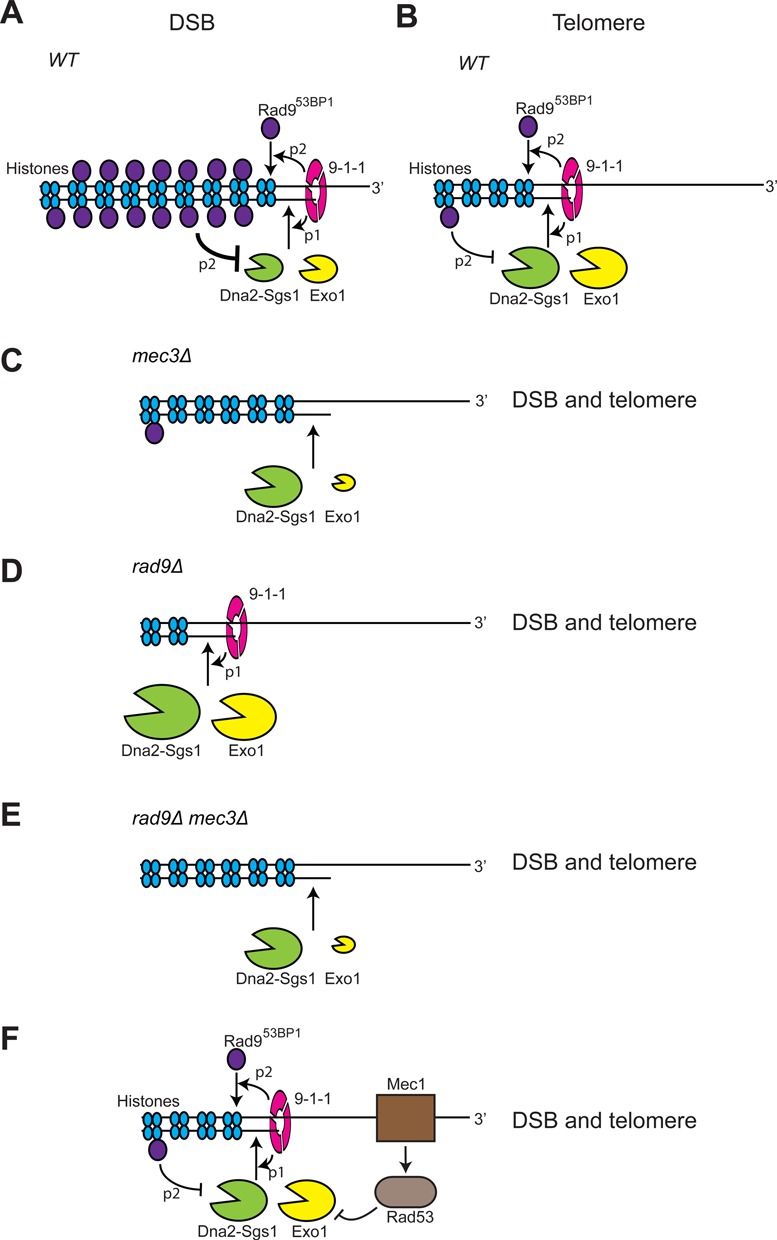
Control of resection at DSBs and telomeres. Models for the roles of 9-1-1, Rad953BP1, Exo1 and Dna2-Sgs1 on resection near DSBs and at uncapped telomeres. The size of the nucleases in each schematic indicates relative resection activities and is deduced by ssDNA measurements in the different genetic settings. Data supporting these figures are taken from Figures 1–7 and Ngo et al. (24). (A, B) 9-1-1 stimulates recruitment of Exo1 and Dna2-Sgs1 to facilitate resection (pathway 1, p1). 9-1-1 stimulates recruitment of Rad953BP1 to inhibit resection (pathway 2, p2). Rad953BP1 binds more near DSBs than uncapped telomeres. (C) In mec3Δ cells, there is less Rad953BP1 recruitment (lack of p2), but there is no 9-1-1 to stimulate activity of Exo1 and Dna2-Sgs1 (lack of p1). At DSBs Exo1 is less active (lack of p1) but Dna2-Sgs1 is more active (lack of p2) (C compared to A). The overall effect is increased resection in mec3Δ mutants (C compared to A). At telomeres Exo1 is less active (lack of p1) but Dna2-Sgs1 activity remains little changed because little Rad953BP1 binds (p2 less active at telomeres), and so the overall effect of mec3Δ is reduced resection (C compared to B). (D) In rad9Δ cells, there is no Rad953BP1 recruitment. Therefore, Dna2-Sgs1 and Exo1 are more active than in (A, B). Dna2-Sgs1 is more active than Exo1 in the absence of Rad953BP1. (E) In rad9Δ mec3Δ cells, there is no 9-1-1 to stimulate Exo1 or Dna2-Sgs1. Therefore, Dna2-Sgs1 and Exo1 are less active than in D. Exo1 is more dependent on 9-1-1 than Dna2-Sgs1. (F) Mec1ATR also initiates a checkpoint cascade to inhibit Exo1.
