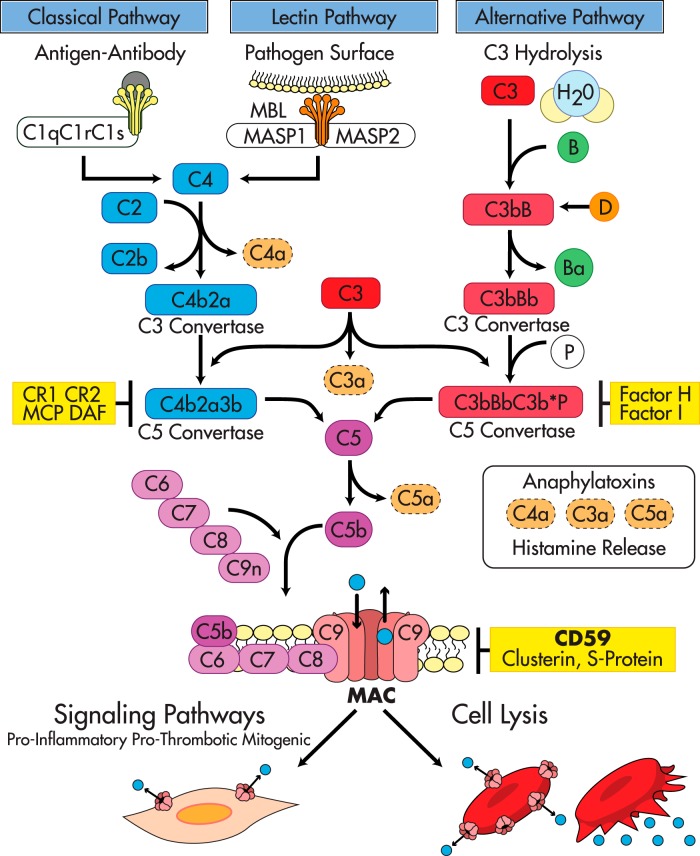
Figure 1.
The complement activation pathways and their regulators. The Complement System: The classical pathway is initiated by immune complexes that activate C1. Activated C1 then recruits C4 and C2 to form a C3-convertase (C4b2a) that fragments C3 into C3a, an anaphylatoxin, and C3b, a molecule at the core of the complement system that binds covalently to hydroxyl groups on carbohydrates or proteins in bacterial surfaces and/or cell membranes. C3b tags invading microorganisms for opsonization and also amplifies the activation cascade acting as a focal site for further complement activation. The phylogenetically older alternative pathway is activated by low-grade cleavage of C3 in plasma; the resulting C3b binds factor B, a protein homologous to C2, to form the C3bB complex. Bound to C3b, factor B is cleaved by the activator factor D, which circulates in an active form as a serine esterase to form C3bBb, the C3-convertase of the alternative pathway. Because basal “tick over” deposition of C3b occurs in all cells exposed to complement, C3b is always available to prime the alternative pathway. However, continued activation and amplification are normally restricted because cell membranes do not bind factor B efficiently, and the inhibitory factor H prevents the association of factor B with C3b. Amplification of the alternative pathway occurs when factor H binds to foreign carbohydrates leaving factor B free to complex with C3b. Specificity for activation of the alternative pathway resides in the ability of factor H to discriminate between “self” and foreign carbohydrate determinants (30, 31, 67). The MBL pathway is initiated when the plasma MBL protein, structurally similar to C1q (165), in a complex with the MASP1 and MASP2 binds to an array of mannose groups on the surface of microorganisms. This binding activates MASPs, which then cleave C2 and C4 leading to the formation of the C3-convertase C4b2a in a manner comparable to activated C1 (30). Terminal complement pathway: The three complement activation pathways eventually converge at the level of C3 with formation of C3b and a C5 convertase that cleaves C5 into C5a, an anaphylatoxin, and C5b. This is the last enzymatic step of the activation cascades; thereafter, the complement pathways share a common sequence through the terminal components C6, C7, C8, and C9, leading to the generation of the MAC, the main effector of complement-mediated tissue damage. The terminal complement components—C6 through C9—necessary to form the MAC are constitutively present in plasma. Formation of the MAC is initiated by C5b, followed by the sequential association of C6 into C5b6, and then C7, C8, and C9. C5b6 is a stable protein that binds to C7 to form the C5b-7 complex that inserts into the lipid bilayer of the plasma membrane. Membrane-bound C5b67 exposes a binding site for C8 leading to formation of the C5b-8 complex, which functions as a docking site for C9. A single C9 first binds to C8 in the C5b-8 complex; then C9 polymerizes, forming the C5b-9 complex known as the MAC. The MAC is a circular polymer of a variable number of C9 monomers (166–170) with the capacity to insert into cell membranes (171) and form a transmembrane pore with hydrophobic domains on the outside and hydrophilic domains in the inside and an effective internal radius of 5–7 nm (172–174). Restriction of complement activity by complement regulators: In the fluid phase, the C1 inhibitor regulates the classical pathway by targeting C1, factors H and I regulate the alternative pathway, and S-protein, clusterin, and serum lipids compete with membrane lipids for reacting with nascent C5b67 (29). In addition to these fluid phase inhibitors, several membrane proteins, DAF (175), membrane cofactor (MCP) (176, 177), and complement receptors 1 and 2 (CR1, CR2) regulate the C3-convertases (178), a major amplification step in the early complement activation cascade, whereas clusterin (179), S-protein (180), and CD59 inhibit formation of the MAC (115, 116, 181–185). Massive or unrestricted activation of complement and MAC formation leads to cell lysis. In the context of reduced restriction, just basal (tick over) complement activity is sufficient to form transient MAC pores in nucleated cells leading to influx and efflux of ions and macromolecules, and activation of cellular pathways that contribute to proliferation, inflammation and thrombosis.