Figure 3.
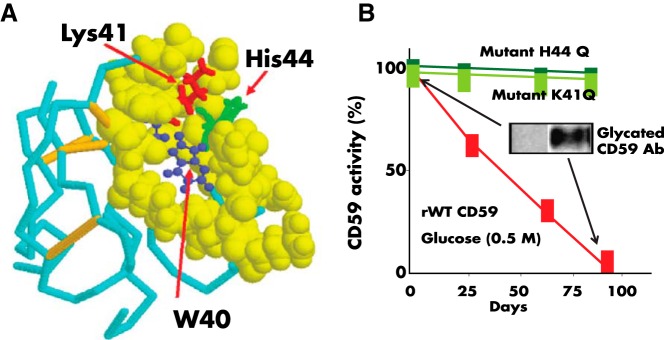
A, Nuclear magnetic resonance structure of human CD59 (from Ref. 114). The figure highlights the Lys41 (red) and His44 (green) amino acid residues that conform the glycation motif and the W40 (blue) residue, a conserved amino acid critical for CD59 activity. The His44 of human CD59 is not found in CD59 from any other species sequenced to date. B, Glycation inactivates human CD59: the activity of recombinant human CD59 was measured in a hemolytic assay using guinea-pig erythrocytes exposed to human MAC formed with purified terminal complement components, as reported in Ref. 8. Incubation of CD59 in the presence of glucose progressively inhibits CD59 activity (red line). Inhibition of activity is associated with glycation of CD59 because: 1) parallel incubation of the same preparation with the nonglycating sugar sorbitol did not affect CD59 activity (see Figure 2 in Ref. 8), and an antibody specific for the glycated form of CD59 showed the presence of glycated CD59 in the protein inactivated after incubation with glucose (inset). Site-directed mutagenesis of either K41 or H44 generated two CD59 mutants (mutant K41Q and mutant H44Q) that were active against human MAC but not inhibited by exposure to glucose.
