Figure 5. Evolutionary dynamics in carcinogenesis (redrawn from Gillies et al., ref. 17).
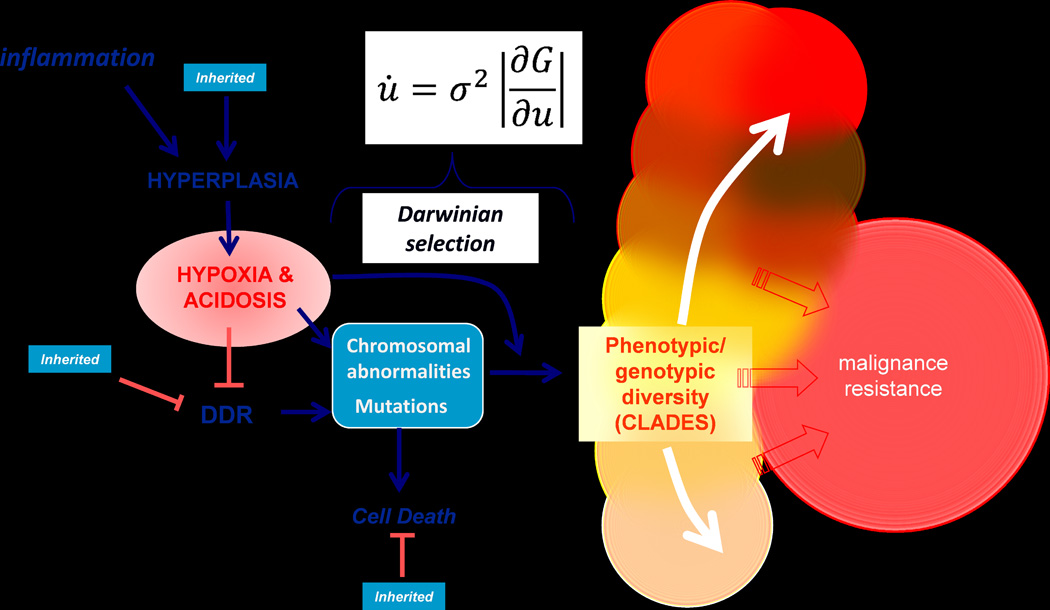
Hyperplasia can be induced by chronic inflammation, and this results in volumes that are hypoxic and acidic (see figures 3 and 4). Through mechanisms that include reactive oxygen (ROS) generated genotoxicity and inhibition of DNA damage response (DDR) pathways, hypoxia and acidosis are known to induce mutations and chromosomal abnormalities, which normally result in apoptotic cell death. With loss of apoptosis, these genomic changes are expected to generate increased phenotypic variance (σ in equation). In addition, hypoxia and acidosis impart strong selection pressure on the emerging phenotypes (∂G/∂μ in equation). These processes combine to generate a high rate of evolution (∂μ/∂t), generating multiple successful clades of cells, each adapted to be maximally fit in their unique microenvironmental niches. The preponderance of these clades is synonymous with malignance and resistance. Notably, many components of this process can be inherited, such as inflammation-independent hyperplasia, reduced DDR, or resistance to apoptosis.
