Abstract
Background
Although some smokers seem interested in obtaining personal lung cancer risk information based on genetic testing for common genetic variants, it is unclear whether or how they understand the predictive power of these tests prior to decision-making. Young smokers are a particularly important group to consider because they are even more likely than other smokers to engage in optimistic biases about their disease risks. We, therefore, assessed how young smokers interpret information about genetic testing for a common lung cancer-associated genetic variant, and whether perceived magnitude of risk conferred by the variant predicted interest in testing.
Methods
Participants (128 smokers recruited at a university) read information about genetic testing for a common lung cancer-associated genetic variant, including that the adverse genotype increased lung cancer risk by about 20%, and that the absolute lung cancer risk was about 11% (`higher risk' genotype) versus about 9% (`lower risk' genotype). We assessed risk interpretation in 2 ways.
Results
On the 5-point measure of risk interpretation, most participants (89%) reported that the genetic variant `increases risk a little.' On the 7-point measure, over a third perceived it to increase risk `a moderate amount' (34%). Interest in testing was quite high (mean = 5.2 on 7-point scale), and was not associated with interpretation of disease risk conferred by the gene variant.
Conclusions
The findings contribute to growing evidence that some young smokers appear interested in personal genetic risk information even when the predictive power is low. The finding that a significant proportion of young smokers interpreted a relative risk of about 20% as `a moderate amount' challenges current assertions in the genomics community that common variants associated with risks of this magnitude have little to no meaning to individuals.
Keywords: genetic testing, genetics, lung cancer, risk, risk perception, smoking
Introduction
Knowledge regarding the modest individual contributions of common genetic variants to complex diseases is increasing [1, 2]. Although not yet used clinically, several direct-to-consumer genetic testing companies have marketed personal genetic tests regarding disease risk, often suggesting the results will empower individuals to make risk-reducing lifestyle changes. These direct-to-consumer genetic tests have also often been offered without the involvement of health professionals [3]. A number of direct-to-consumer genetic testing products include genetic information about smoking-related diseases such as lung cancer. In surveys, consumers in general seem interested in receiving personal genetic information based on low-penetrance DNA variants [4], and smokers specifically seem interested in genetic information about smoking-related diseases such as lung cancer [5]. One study found high levels of interest in genetic testing for smoking-related diseases specifically among young college-age smokers [6]. On the one hand, this interest could be a good thing. Young smokers, compared with older smokers, may particularly benefit from genetic risk information because of the greater potential of early intervention to reduce future disease risk. On the other hand, smokers in general, and younger smokers in particular, are prone to optimistic biases about their future disease risk [7]. Although smokers acknowledge they are at increased risk of disease compared to nonsmokers, they show biases in that they tend to believe they are at lower risk than other smokers [8]. Genetic test results indicating even slight decreased or increased risks of smoking-related diseases could backfire and further support continued smoking in young smokers.
An important first step in predicting how young smokers are likely to respond to genetic test results for smoking-related diseases is to understand how they interpret information generally about genetic variants modestly associated with risk of smoking-related diseases such as lung cancer. Whether young smokers, or consumers more generally, understand the low predictive power of such genetic tests prior to deciding to move forward with testing is unknown. Evidence suggests the public and healthcare providers have limited understanding of genetic underpinnings of common diseases [2, 9] and risk [10], raising questions about the extent to which individuals make sense of what the information means for their health and whether they are making genuinely informed decisions about testing [11].
Studies examining downstream behavioral effects of personal genetic risk information have tended to focus on smoking cessation and lung cancer risk [12–16], several testing for GSTM1 [14–16]. The GSTM1 gene is absent in ~50% of the population, and estimates suggest people homozygous for null allelic state (GSTM1-null) have a ~17% increased risk for lung cancer (OR = 1.17; 95% CI = 1.07–1.27) [17]. As with commercial genetic testing, however, researchers know little about how smokers in these early studies interpreted the baseline information they received regarding the risk conferred by the GSTM1 adverse genotype.
Our overarching aim in the present study was, therefore, to assess how young smokers would interpret the risk associated with a common lung cancer-associated genetic variant, GSTM1-null, and to examine whether interest in testing was associated with perceived magnitude of risk conferred by the variant. We sampled college-age smokers recruited in a university setting because this group was the target audience for a subsequent trial of lung cancer risk genetic testing due to their higher literacy and lower motivation to quit smoking relative to other smokers [18]. Our study addressed 4 questions:
-
(1)
How do young smokers interpret the risk associated with a common lung cancer-associated gene variant of low penetrance?
-
(2)
Do they consider personal genetic information for lung cancer susceptibility to be personally relevant and are they interested in genetic testing for lung cancer susceptibility?
-
(3)
Do they evaluate the information as credible, trustworthy, useful, and nondirective?
-
(4)
What factors predict interest in genetic testing for lung cancer risk among these young smokers?
We predicted (a) that this sample of young smokers would interpret the gene variant as only influencing lung cancer risk by a small amount, and explored the hypothesis that they would (b), therefore, consider genetic testing for lung cancer susceptibility to be irrelevant and would express little interest in undergoing genetic testing for the variant overall. We hypothesized that the information would (c) be perceived as credible, trustworthy, useful, and nondirective, and that (d) the perceived magnitude of risk conferred by the genotype would correlate positively with interest in genetic testing. We also hypothesized that worry about lung cancer and motivation to quit smoking would correlate positively with interest in testing, based on previous research [19].
Methods
Recruitment and Procedure
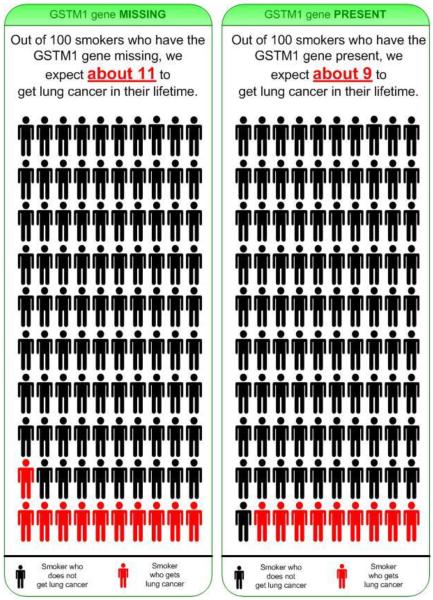
As described elsewhere [20, 21], participants were smokers aged 18–21 years who attended the University of Florida. The inclusion criteria were being a student at the University of Florida, aged 17–22 years, having smoked at least 1 cigarette in the prior week and at least 50 cigarettes in their lifetime. We used active and passive recruitment methods. Potential participants were approached on campus, and via advertisements that indicated they would receive $ USD 40 for participation. Interested individuals received a web link where they completed a brief screener at home (n = 409). Eligible smokers who consented completed a baseline online survey (n = 182). Following completion, they were contacted and invited to attend another session in the psychology laboratory (n = 128). At the laboratory, participants read an online brochure adapted from previous research [14, 15] describing GSTM1 genetic testing for lung cancer susceptibility. In order to simplify the risk communication message, we rounded the GSTM1-null 1.17 OR associated with lung cancer risk up to 1.2 (i.e. we rounded the estimated 17% relative risk up to 20%). In addition to informing participants that the risk variant was associated with a 20% increased relative risk of lung cancer, we also provided the absolute risk estimates of about 9% lifetime risk for GSTM1 present and about 11% lifetime risk for GSTM1 missing. Figure 1 shows one of the brochure pages presenting one of the icon arrays depicting risk [21] for more detail regarding the icon arrays used in this study). For the complete brochure, see online supplementary material (www.karger.com/doi/10.1159/000■■■). Afterwards, participants completed another questionnaire online in the laboratory. Most participants completed the laboratory session within 3 weeks of the baseline online survey. The study was approved by the Institutional Review Boards at the University of Florida and Duke University Medical Center.
Fig. 1.
Icon array depicting risk associated with the 2 genotypes. The figure shows one page from the 20-page information brochure that was developed to explain to participants what genetic testing is, what GSTM1 is, and how GSTM1 may be associated with lung cancer risk, as well as the risks, benefits and limitations of GSTM1 genetic testing for lung cancer risk. As the figure illustrates, here participants were informed that absolute risk of developing lung cancer if missing GSTM1 was estimated to be about 11 out of 100 (or 11%), and that absolute risk of developing lung cancer if GSTM1 is present was estimated to be about 9 out of 100 (or 9%).
Measures
Demographics
We collected participants' age, gender, and race/ethnicity.
Family History of Lung Cancer
We assessed family history of lung cancer with the item: `Please check which relatives you have that have had lung cancer'. Participants could then check one or more of 24 options, including: biological relatives such as `brother', `sister', `son', `daughter', `father', `mother', `cousin (female)', `cousin (male)', `grandfather', and `grandmother'; nonbiological relatives including `husband', `wife', `brother-in-law', and `sister-in-law'; and other relationships such as `friend' and `other (please specify)'. We summed all of the biological relatives and categorized participants according to whether they had zero (low family history-based risk), 1 (moderate family history-based risk), or 2 or more (high family history-based risk) biological relatives with lung cancer.
Smoking Characteristics
Motivation to quit smoking (1 = not at all strong, 7 = extremely strong) and worry about lung cancer (1 = never, 7 = more than once a day) were assessed using single items.
Perceived Risk of Lung Cancer
We measured perceived risk of lung cancer (unrelated to GSTM1) in 2 ways. First, we assessed `perceived absolute risk of lung cancer' as follows: `What do you think is your chance of getting lung cancer from your smoking if you don't quit?' (1 = no chance, 7 = certain to happen). Second, we assessed `perceived relative risk of lung cancer' as follows: `Compared to other college smokers your age and sex, what do you think is your chance of getting lung cancer from your smoking if you don't quit?' (1 = much below average, 5 = much above average).
Interpretation of Lung Cancer Risk Conferred by GSTM1
Interpretation of lung cancer risk associated with GSTM1 was assessed on 2 dimensions. The first, `increases-decreases' dimension assessed interpretation of absolute risk for a smoker generally using a 7-point scale: `Having/missing the GSTM1 enzyme… 1 = decreases a smoker's expected risk of getting lung cancer by a lot, 2 = decreases a smoker's expected risk of getting lung cancer by a moderate amount, 3 = decreases a smoker's expected risk of getting lung cancer by a little, 4 = does not affect a smoker's expected risk of getting lung cancer, 5 = increases a smoker's risk of getting lung cancer by a little, 6 = increases a smoker's expected risk of getting lung cancer by a moderate amount, 7 = increases a smoker's expected risk of getting lung cancer by a lot.'
The second, `relative-to-average' approach assessed interpretation of personal risk relative to other smokers using a 5-point scale: `Overall, if you have/are missing the GSTM1 enzyme, what is your risk of getting lung cancer? 1 = much lower than average, 2 = slightly lower than average, 3 = same average risk as others, 4 = slightly higher than average, 5 = much higher than average.'
Assessment of the Information Brochure
We assessed whether participants perceived the information as credible, trustworthy, useful, and understandable with 4 items: `In your opinion, how credible, that is believable, is the information presented to you about genetic testing and lung cancer susceptibility? (1 = not at all credible, 7 = completely credible)'; `In your opinion, how trustworthy is the information presented to you about genetic testing and lung cancer susceptibility? (1 = not at all trustworthy, 7 = completely trustworthy)'; `How useful was the information presented to you about genetic testing and lung cancer susceptibility? (1 = not at all useful, 7 = extremely useful)'; `How understandable was the information presented to you about genetic testing and lung cancer susceptibility? (1 = not at all understandable, 7 = completely understandable)'.
We assessed the perceived adequacy of the information for decision making with an item that read: `Do you feel the information was adequate for people to make decision about genetic testing for lung cancer susceptibility? (yes, somewhat, no)'. We assessed the perceived directiveness of the information with an item that read: `Did you find that the information you reviewed told you whether or not you should be tested? (yes, somewhat, no)'. These items were analyzed separately.
Perceived Personal Relevance
We assessed perceived personal relevance with 6 items from the 10-item Personal Involvement Inventory [22]. All items loaded on a single factor with eigenvalues >0.4, Cronbach's alpha = 0.87.
Interest in Testing
We assessed interest in testing for GSTM1 after reading the brochure with a single item (1 = not at all interested, 7 = extremely interested).
Statistical Analyses
We analyzed demographic and smoking characteristics, understandability, credibility, perceived usefulness, personal relevance, and interest in genetic testing using frequencies, means, and SDs. We explored bivariate associations between interest in genetic testing and other variables using Pearson correlations and simultaneous regression analysis. Analyses were conducted using SPSS v.19.0.
Results
Demographic and Smoking-Related Characteristics at Baseline
Descriptive statistics for the participants are published elsewhere [20, 21]; table 1 shows the demographic and additional smoking-related characteristics.
Table 1.
Demographic and smoking-related characteristics at baseline
| Characteristics | n = 128 |
|---|---|
| Gendera | |
| Male | 66 (51.6%) |
| Female | 61 (47.7%) |
| Race/ethnicity | |
| White or Caucasian (not Hispanic) | 91 (71.1%) |
| Hispanic | 23 (18.0%) |
| African American or Black (not Hispanic) | 4 (3.1%) |
| Asian or Pacific Islander | 4 (3.1%) |
| Other/mixed ethnicity | 6 (4.7%) |
| Family members with lung cancerb, relatives | |
| 0 | 76 (59.3%) |
| 1 | 34 (26.6%) |
| ≥2 | 18 (14.1%) |
|
| |
| Age | 19.9±1.0 (18–22) |
| Years of smoking | 2.6±1.6(0–7) |
| Number of quit attempts in the last year | 24.4±49.2 (0–360) |
| Desire to quit smoking (1 = not at all strong, 7 = extremely strong) | 3.9± 1.72 (1–7) |
| Worry about lung cancer (1 = never, 7 = more than once a day) | 2.4±1.17(l–6) |
| Perceived absolute risk of lung cancer (1 = no chance, 7 = certain to happen) | 4.4± 1.24 (1–7) |
| Perceived comparative risk of lung cancer (1 = much below average, 5 = much above average) | 2.9±0.90 (1–5) |
Due to incomplete data from one participant, the numbers do not sum to 100%.
Assessed after brochure was presented (all other measures were assessed before).
Figures are numbers with percentages or mean ± SD with ranges in parentheses.
Interpretation of Disease Risk Conferred by the Common Gene Variant
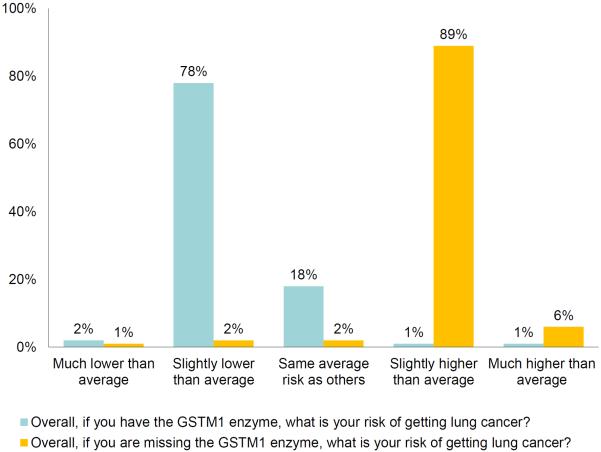
In regards to our first research question, when interpretation was assessed using the 5-point `relative-to-average' measure, 89% of the participants agreed their personal lung cancer risk would be `slightly higher than average' if they were GSTM1-missing; 78% agreed their lung cancer risk would be `slightly lower than average' if GSTM1-present (fig. 2). Mean response for the latter item (mean = 2.2, SD = 0.52) was significantly lower than the scale midpoint (95% CI = −0.90 to −0.71, t = 17.57; p < 0.001), whereas mean response for the former item (mean = 4.0, SD = 0.47) was significantly higher than the scale midpoint (95% CI = 0.90–1.07, t = 23.73; p < 0.001).
Fig. 2.
Interpretation of lung cancer risk conferred by GSTM1 to participant compared to others on 5-point scale. The majority of participants interpreted `GSTM1 present' to mean that risk of developing lung cancer is `slightly lower than average'. The majority of participants interpreted `GSTM1 missing' to mean that risk of developing lung cancer is `slightly higher than average'.
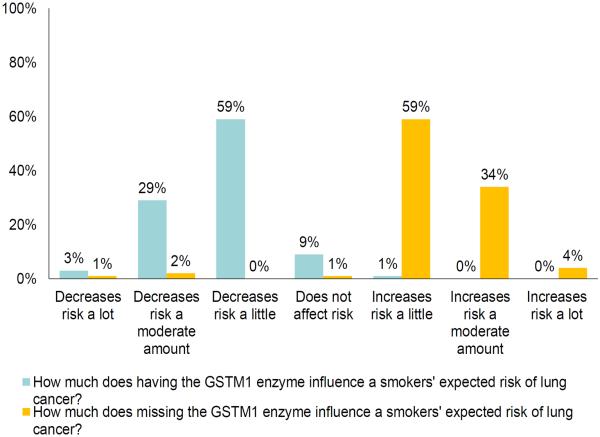
When assessed using the 7-point `increases-decreases' measure, 59% of the participants agreed GSTM1-missing `increases risk a little,' and 59% agreed GSTM1-present `decreases risk a little' (fig. 3). As figure 3 shows, a third (34%) of participants agreed GSTM1-missing `increases risk a moderate amount.' Mean response to the first item (mean = 5.3, SD = 0.81) was significantly higher than the scale midpoint (95% CI = 1.19–1.47, t = 18.45; p < 0.001). Mean response to the second item (mean = 2.6, SD = 0.69) was significantly lower than the scale midpoint (95% CI = −1.37 to −1.13, t = 20.58; p < 0.001).
Fig. 3.
Interpretation of lung cancer risk conferred by GSTM1 to general smoker on 7-point scale. This figure illustrates that 59% of participants interpreted the information they received to mean that having GSTM1 `decreases risk a little', and that 29% interpreted it to mean that having GSTM1 present `decreases risk a moderate amount'. It also illustrates that 59% of participants interpreted the information to mean that missing GSTM1 `increases risk a little', and that 34% interpreted it to mean that missing GSTM1 `increases risk a moderate amount'.
Although responses to the 4 interpretation items differed significantly from the scale midpoint, the pattern of responses displayed in figures 2 and 3 suggests that most participants interpreted that the GSTM1-null gene variant influenced the disease risk only a small amount, thus supporting hypothesis 1.
Perceived Personal Relevance of the Information and Interest in Testing
In regards to our second research question, participants on average perceived the information to be personally relevant (mean = 5.8, SD = 1.1) and were interested in genetic testing (mean = 5.2, SD = 1.66, range 1–7). Both means were greater than the scale midpoint (p < 0.0001), providing no evidence to support hypothesis 2.
Assessment of the Information Brochure
In regards to our third question, participants on average indicated they perceived the genetic information to be credible (mean = 6.0, SD = 0.9), trustworthy (mean= 5.7, SD = 1.2), useful (mean = 5.7, SD = 1.2), and understandable (mean = 6.6, SD = 0.7); all means were greater than the scale midpoint (all p < 0.0001). These findings support hypothesis 3.
Sixty-seven percent of participants thought the information was adequate for decision making and another 30% thought it was somewhat adequate. Believing the information was adequate for decision making correlated with believing the information was credible (r = 0.35, p < 0.001), trustworthy (r = 0.34, p < 0.001), useful (r = 0.24, p < 0.006), understandable (r = 0.27, p < 0.003), personally relevant (r = 0.30, p < 0.001), and with interest in genetic testing (r = 0.19, p < 0.05).
Of note, participants tended to perceive that the information in the brochure was telling them whether they should have genetic testing: 37% responded `yes' the information told them whether to be tested, 43% said `somewhat', and 20% `no'.
Factors Associated with Interest in Genetic Testing
We found no support for hypothesis 4. Interest in genetic testing was not associated with interpretations of risk conferred by the gene variant, but was associated with perceived relevance and usefulness of the information, and with worry (table 2). Worry about lung cancer correlated with having a family history of lung cancer and perceived relevance of the information. Because of the shared variance between the various predictors of interest in getting a genetic test, we entered all variables significant at p < 0.05 in table 2 as predictors of interest in getting a genetic test in a simultaneous regression analysis. Only one variable – relevance – emerged as a significant predictor of interest, b = 0.14, SE = 0.02, t = 6.67, p < 0.0001. Further probing revealed that relevance accounted for 31% of the variance in participants' interest in getting a genetic test. The other variables collectively accounted for only 1.5% additional variance.
| Interest in testing | Adequacy of information | Personal relevance | Family history | Desire to quit | Worry | Time as a smoker | Risk with GSTM1 present | Risk with GSTM1 absent | Number of quit attempts | Absolute risk | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Interest in testing | – | ||||||||||
| Adequacy of information for decision making | 0.19* | – | |||||||||
| Family history of lung cancer | 0.15 | −0.09 | – | ||||||||
| Personal relevance of information | 0.55*** | 0.30*** | 0.11 | – | |||||||
| Desire to quit smoking | 0.03 | 0.16 | 0.02 | 0.05 | – | ||||||
| Worry about lung cancer | 0.19* | 0.06 | 0.21* | 0.21* | 0.10 | – | |||||
| Length of time as a smoker | 0.03 | −0.06 | 0.08 | 0.09 | 0.01 | −0.02 | – | ||||
| Number of quit attempts | −0.12 | 0.15 | 0.02 | −0.11 | 0.08 | −0.07 | 0.18* | – | |||
| If GSTM1-present, what is personal risk relative to someone with GSTM1-absent | 0.09 | 0.00 | −0.07 | 0.03 | −0.04 | 0.01 | −0.13 | 0.05 | |||
| If GSTM1-absent, what is personal risk relative to someone with GSTM1-present | 0.03 | 0.04 | −0.12 | 0.06 | 0.03 | −0.05 | 0.01 | 0.08 | 0.14 | ||
| Absolute risk for lung cancer | 0.13 | 0.03 | 0.03 | 0.15 | 0.31*** | 0.32*** | −0.03 | −0.12 | 0.02 | 0.12 | – |
| Comparative risk for lung cancer | 0.15 | −0.18 | 0.06 | 0.04 | 0.05 | 0.21* | −0.02 | −0.24** | −0.07 | 0.03 | 0.30*** |
p < 0.05,
p < 0.01,
p < 0.001.
Discussion
In this study, we examined young smokers' responses to an information brochure that described in detail the lung cancer risk estimated to be associated with a common genetic variant. The majority of participants appeared to interpret the lung cancer risk conferred by the GSTM1 variant as `small' on one measure, but over a third interpreted the risk conferred by the variant as `moderate' on another. Participants perceived the information to be credible, trustworthy, useful, and understandable, although a third perceived it to be directive. Interest in being testing for GSTM1 was quite high, and not associated with perceptions of lung cancer risk conferred by the gene variant.
The finding that a substantial proportion of participants interpreted an increase of about 20% in relative terms, and from about 9–11% lifetime risk in absolute terms, as a `moderate' increase in risk on one of the measures of risk interpretation, was something of a surprise and could be a reflection of poor understanding of risk information among the smokers in this study. Even healthcare providers have limited understanding of genetics [2, 9] and low probabilities [10]. An alternative explanation is that the participants focused on the gist of the information presented in the brochure – that having the gene was `good,' whereas not having the gene was `bad' – and that under these circumstances interpreting the risk as `moderate' is not in fact surprising or unreasonable. This latter interpretation challenges current notions in the genomics community that ORs in the realms of 1.2, or relative risks of about 20%, are too small to have meaning for individuals. Either way, the finding highlights the need for further work understanding how people interpret small increases or decreases in genetic risk, and how to effectively communicate such risk information.
Nondirectiveness is a guiding principle for genetic counseling [23], and it is, therefore, arguably problematic that a substantial minority of participants felt the information presented in the brochure was directive. While important in promoting autonomy in genetic counseling contexts such as reproductive decision making, it has been argued that nondirectiveness is of less importance in emerging areas of genetic counseling such as those that focus on lifestyle behavior changes [23], and so being directive in the present context of nicotine smoking could be defensible. Even here, however, evidence suggests that being directive could be undesirable, because it could elicit `psychological reactance' [24]. The theory of psychological reactance posits that young smokers could respond to efforts to `tell them what to do' by showing the opposite behavior in an attempt to reassert their freedom [24]. As genomics is increasingly considered in the context of preventive lifestyle behavior change interventions, it will be important to consider further the extent to which directiveness or nondirectiveness is seen as a guiding principle for genetic risk communications.
Our findings have implications for how the understanding of genetic risk is assessed in future studies. Two different measures of interpretation of risk led to different outcomes – whereas the 5-point measure suggested participants `got' that the gene variant was associated with only a `slightly higher' than average disease risk, the 7-point measure showed more sensitivity and many participants interpreted the information to mean the variant increased risk `by a moderate amount.' This greater sensitivity was most likely due to the larger number of response options provided, but this requires confirmation in future work.
Previous research suggests a major reason people generally are interested in personal genetic information is curiosity [4], and we have previously demonstrated this in our sample of young smokers [20]. Our finding in the present study that there was no association between interpretation of the risk information presented and interest in testing build on this notion by suggesting interest is not fundamentally based on misunderstanding or overinterpreting the strength of disease risk conferred by the gene variant.
Limitations included that the information brochure we used to educate participants about the genetic test was text-heavy and long. It was designed for use in a subsequent trial in which young smokers were offered GSTM1 genetic testing, and so went into considerable depth about this one gene variant and disease association. However, as whole genome sequencing replaces single variant genetic tests, it will no longer be feasible to go into such depth explaining every possible result that might be returned to the individual. Researchers will need to develop new multimedia approaches to explaining potential genetic results to help individuals make informed decisions about genomic testing or sequencing in the future. Also, the fact that our sample comprised college-age smokers limits the generalizability of our findings. Finally, we presented the genetic information within the context of a research study with university students, which could have influenced the students' ratings of the credibility and trustworthiness of the information provided in the brochure.
Despite the limitations, this study is unique in that researchers have devoted little effort to developing and evaluating decision aids for young smokers or people more generally who are considering personal genetic testing or sequencing. Our findings contribute to growing evidence that smokers are interested in personal genetic risk information even when they are aware of the low utility for disease risk prediction, and highlight considerations regarding how smokers interpret risk information they receive in educational materials. The findings are relevant to interventionists in clinical practice or public health because they suggest that even when the risk associated with genetic variation appears to be low in absolute terms, a considerable number of smokers may nonetheless interpret their risk feedback as meaningful information. Going forward, more work is needed to develop effective decision support tools to help smokers and nonsmokers make informed decisions in the context of genetic testing or sequencing that involves variants of low penetrance.
Acknowledgement
This research was partially funded by a grant from the National Cancer Institute (R01 CA121922-01A2).
References
- 1.Green ED, Guyer MS. National Human Genome Research Institute: Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470:204–213. doi: 10.1038/nature09764. [DOI] [PubMed] [Google Scholar]
- 2.Guttmacher AE, Porteous ME, McInerney JD. Educating health-care professionals about genetics and genomics. Nat Rev Genet. 2007;8:151–157. doi: 10.1038/nrg2007. [DOI] [PubMed] [Google Scholar]
- 3.Myers MF, Bernhardt BA. Direct-to-consumer genetic testing: introduction to the special issue. J Genet Couns. 2012;21:357–360. doi: 10.1007/s10897-012-9500-3. [DOI] [PubMed] [Google Scholar]
- 4.McGuire AL, Diaz CM, Wang T, Hilsenbeck SG. Social networkers' attitudes toward direct-to-consumer personal genome testing. Am J Bioeth. 2009;9:3–10. doi: 10.1080/15265160902928209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanderson SC, O'Neill SC, Bastian LA, Bepler G, McBride CM. What can interest tell us about uptake of genetic testing? Intention and behavior amongst smokers related to patients with lung cancer. Public Health Genomics. 2010;13:116–124. doi: 10.1159/000226595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McBride CM, Lipkus IM, Jolly D, Lyna P. Interest in testing for genetic susceptibility to lung cancer among Black college students `at risk' of becoming cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2005;14:2978–2981. doi: 10.1158/1055-9965.EPI-05-0269. [DOI] [PubMed] [Google Scholar]
- 7.Arnett JJ. Optimistic bias in adolescent and adult smokers and nonsmokers. Addict Behav. 2000;25:625–632. doi: 10.1016/s0306-4603(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein ND. Accuracy of smokers' risk perceptions. Ann Behav Med. 1998;20:135–140. doi: 10.1007/BF02884459. [DOI] [PubMed] [Google Scholar]
- 9.Pearl PL, Pettiford JM, Combs SE, Heffron A, Healton S, Hovaguimian A, Macri CJ. Assessment of genetics knowledge and skills in medical students: insight for a clinical neurogenetics curriculum. Biochem Mol Biol Educ. 2011;39:191–195. doi: 10.1002/bmb.20489. [DOI] [PubMed] [Google Scholar]
- 10.Reyna V, Brainerd C. Numeracy, ratio bias, and denominator neglect in judgments of risk and probability. Learn Individ Differ. 2008;18:89–107. [Google Scholar]
- 11.Michie S, Dormandy E, Marteau TM. The multi-dimensional measure of informed choice: a validation study. Patient Educ Couns. 2002;48:87–91. doi: 10.1016/s0738-3991(02)00089-7. [DOI] [PubMed] [Google Scholar]
- 12.Ito H, Matsuo K, Wakai K, Saito T, Kumimoto H, Okuma K, Tajima K, Hamajima N. An intervention study of smoking cessation with feedback on genetic cancer susceptibility in Japan. Prev Med. 2006;42:102–108. doi: 10.1016/j.ypmed.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Lerman C, Gold K, Audrain J, Lin TH, Boyd NR, Orleans CT, Wilfond B, Louben G, Caporaso N. Incorporating biomarkers of exposure and genetic susceptibility into smoking cessation treatment: effects on smoking-related cognitions, emotions, and behavior change. Health Psychol. 1997;16:87–99. doi: 10.1037//0278-6133.16.1.87. [DOI] [PubMed] [Google Scholar]
- 14.McBride CM, Bepler G, Lipkus IM, Lyna P, Samsa G, Albright J, Datta S, Rimer BK. Incorporating genetic susceptibility feedback into a smoking cessation program for African-American smokers with low income. Cancer Epidemiol Biomarkers Prev. 2002;11:521–528. [PubMed] [Google Scholar]
- 15.Sanderson SC, Humphries SE, Hubbart C, Hughes E, Jarvis MJ, Wardle J. Psychological and behavioural impact of genetic testing smokers for lung cancer risk: a phase II exploratory trial. J Health Psychol. 2008;13:481–494. doi: 10.1177/1359105308088519. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson SC, O'Neill SC, White DB, Bepler G, Bastian L, Lipkus IM, McBride CM. Responses to online GSTM1 genetic test results among smokers related to patients with lung cancer: a pilot study. Cancer Epidemiol Biomarkers Prev. 2009;18:1953–1961. doi: 10.1158/1055-9965.EPI-08-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benhamou S, Lee WJ, Alexandrie AK, Boffetta P, Bouchardy C, Butkiewicz D, Brockmüller J, Clapper ML, Daly A, Dolzan V, Ford J, Gaspari L, Haugen A, Hirvonen A, Husgafvel-Pursiainen K, Ingelman-Sundberg M, Kalina I, Kihara M, Kremers P, Le Marchand L, London SJ, Nazar-Stewart V, Onon-Kihara M, Rannug A, Romkes M, Ryberg D, Seidegard J, Shields P, Strange RC, Stücker I, To-Figueras J, Brennan P, Taioli E. Meta- and pooled analyses of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancer risk. Carcinogenesis. 2002;23:1343–1350. doi: 10.1093/carcin/23.8.1343. [DOI] [PubMed] [Google Scholar]
- 18.McBride CM, Bowen D, Brody LC, Condit CM, Croyle RT, Gwinn M, Khoury MJ, Koehly LM, Korf BR, Marteau TM, McLeroy K, Patrick K, Valente TW. Future health applications of genomics: priorities for communication, behavioral, and social sciences research. Am J Prev Med. 2010;38:556–565. doi: 10.1016/j.amepre.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanderson SC, Wardle J. Associations between anticipated reactions to genetic test results and interest in genetic testing: will self-selection reduce the potential for harm? Genet Test. 2008;12:59–66. doi: 10.1089/gte.2007.0047. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill SC, Lipkus IM, Sanderson SC, Shepperd J, Docherty S, McBride CM. Motivations for genetic testing for lung cancer risk among young smokers. Tob Control. 2013;22:406–411. doi: 10.1136/tobaccocontrol-2011-050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepperd JA, Lipkus IM, Sanderson SC, McBride CM, O'Neill SC, Docherty S. Testing different communication formats on responses to imagined risk of having versus missing the GSTM1 gene. J Health Commun. 2013;18:124–137. doi: 10.1080/10810730.2012.688245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaichkowsky JL. The personal Involvement Inventory: Reduction, Revision, and Application to Advertising. Journal of Advertising. 1994;23:59–70. [Google Scholar]
- 23.Biesecker BB. Goals of genetic counseling. Clin Genet. 2001;60:323–330. doi: 10.1034/j.1399-0004.2001.600501.x. [DOI] [PubMed] [Google Scholar]
- 24.Brehm SS, Brehm JW. Psychological Reactance: A Theory of Freedom and Control. Academic Press; New York: 1981. [Google Scholar]