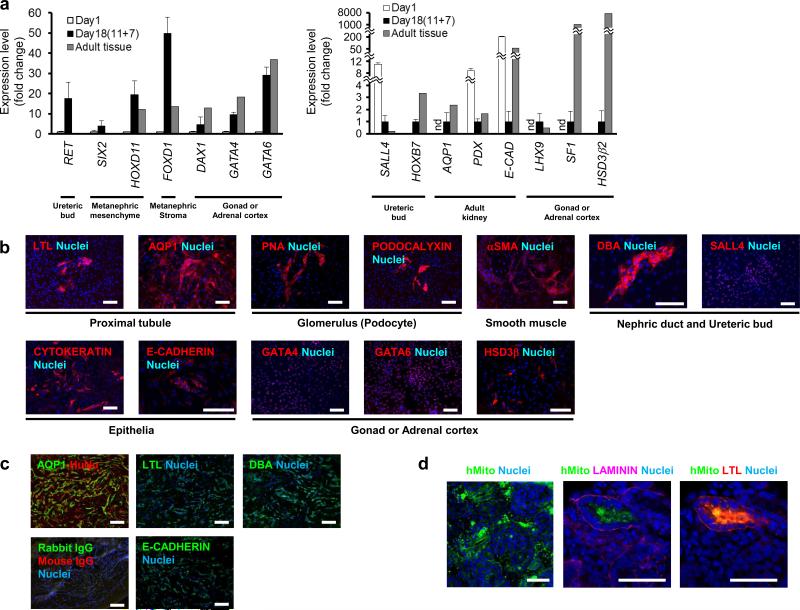
Figure 7.
In vitro and in vivo differentiation of OSR1+ cells into IM derivatives. (a) The expression of marker genes for the developing kidney, gonad and adrenal cortex in the differentiated cells on culture day 18 (after an additional 7-day culture of the day 11 isolated OSR1+ cells differentiated from an OSR1-GFP knock-in hiPSC line, 3D45, using the single cell method). The mRNAs of human adult kidney were used as controls for the expression of RET, SALL4, HOXB7, SIX2, HOXD11, FOXD1, AQP1, PODOCALYXIN and E-CADHERIN. The mRNAs of human adult testis were used as controls for DAX1 and LHX9. The mRNAs of human adult ovary were used as controls for GATA4 and GATA6. The mRNAs of human adult adrenal gland were used as controls for SF1 and HSD3β2. Each value of RET, SIX2, HOXD11, FOXD1, DAX1, GATA4 and GATA6 was normalized to the samples on day 1 before treatments, while that of SALL4, HOXB7, AQP1, PODOCALYXIN, E-CADHERIN, LHX9, SF1 and HSD3β2 was normalized to the samples on day18, whose expression levels in the day 1 samples were so low or much higher than day 18 samples or adult tissues. The data from three independent experiments are presented as the means ± SD (n=3). nd; not detected. PDX; PODOCALYXIN, E-CAD; E-CADHERIN. (b) The differentiated cells on day 18 were stained with antibodies or lectins against markers for IM derivatives: LTL and AQP1 for proximal renal tubule, PNA and PODOCALYXIN for glomerular podocytes, αSMA for smooth muscle, DBA and SALL4 for nephric duct and ureteric bud, epithelial markers CYTOKERATIN and E-CADHERIN, and GATA4, GATA6 and HSD3β for the gonads or adrenal cortex. (c) AQP1 and human nuclear antigen (HuNu), control, LTL, E-CADHERIN and DBA staining of histological sections of 4-week-old hiPSC-derived IM grafts transplanted into immunodeficient mice (NOD. CB17-Prkdcscid/J). (d) Immunostaining of the histological sections of organ culture samples collected on day 7 for human mitochondria (hMito) and for LTL or LAMININ. Note that the right two panels are images of the same section. Scale bars, 50 μm.