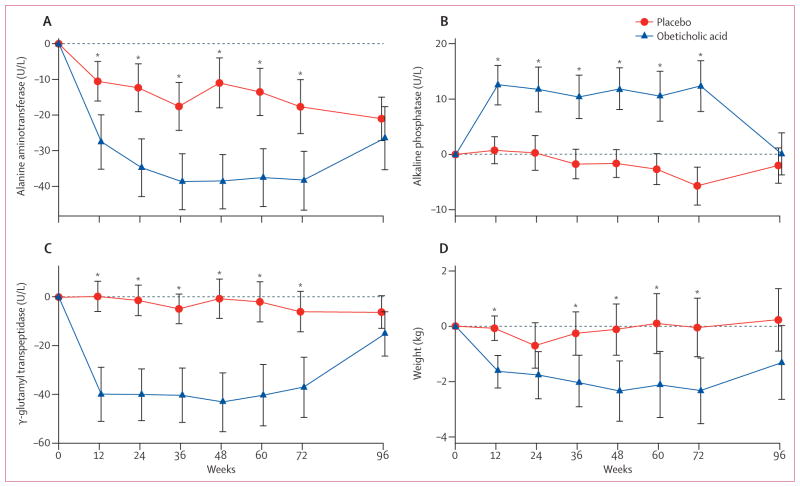
Figure 2. Changes from baseline in liver enzymes and bodyweight according to treatment group.
Mean values of changes from baseline during treatment with obeticholic acid (141 patients) or placebo (142 patients) for up to 72 weeks followed by a 24-week post-treatment period are shown. Error bars show 95% CIs. *p<0·05; p values were derived from linear regression modelling change as a function of treatment group and the baseline value of the outcome. Data are included from patients whose treatment was terminated early according to protocol design and their serum biochemical test results obtained 24 weeks after stopping treatment are included with the 96-week mean values. (A) Alanine aminotransferase concentrations decreased during treatment with obeticholic acid, reaching a reduced baseline 36 weeks after initiating treatment, whereas concentrations in patients treated with placebo remained unchanged. Alanine aminotransferase concentrations in the obeticholic acid group reverted back to being indistinguishable from placebo 24 weeks after treatment discontinuation. (B) Serum alkaline phosphatase concentrations increased whereas (C) serum γ-glutamyl transpeptidase decreased early in the course of treatment with obeticholic acid. (D) Bodyweight decreased throughout treatment with a rebound back toward baseline after treatment discontinuation.