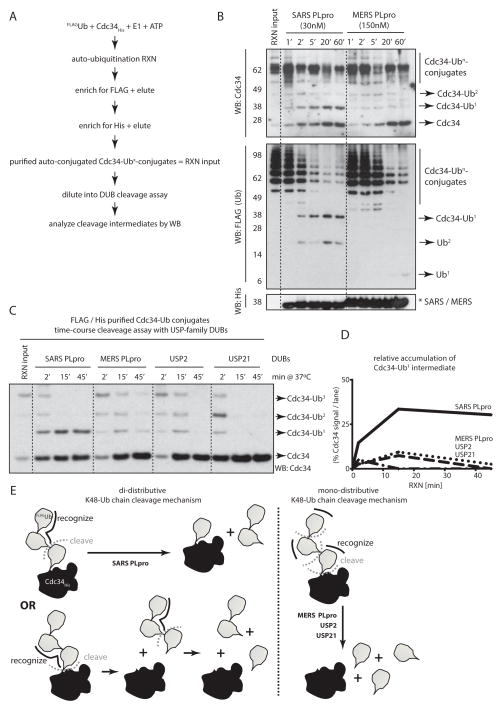
Figure 4. SARS PLpro is a unique “distal di-distributive” deubiquitinating enzyme.
(A) Schematics of the purification strategy of poly-Ub-conjugated Cdc34 in order to asses the directionality of cleavage of Ub chains. (B) Time-course cleavage of HisCdc34-poly-UbFLAG conjugates by SARS and MERS PLpro, revealing stabilization of mono-Ub-conjugated Cdc34 as a cleavage product by SARS PLpro and not by MERS PLpro. (C) Time-course cleavage of HisCdc34-poly-UbFLAG conjugates by USP-family DUBs. (D) Quantification of the relative abundance of the HisCdc34-mono-UbFLAG species from the time-course cleavage assay in (C). (E) Schematic representation of the mono-(for MERS PLpro, USP2 and USP21) and the di-distributive cleavage mechanism (for SARS PLpro), indicating the accumulation of mono-Ub-conjugated substrates only in the case of the di-distributive cleavage mechanism displayed by SARS PLpro.