Figure 7.
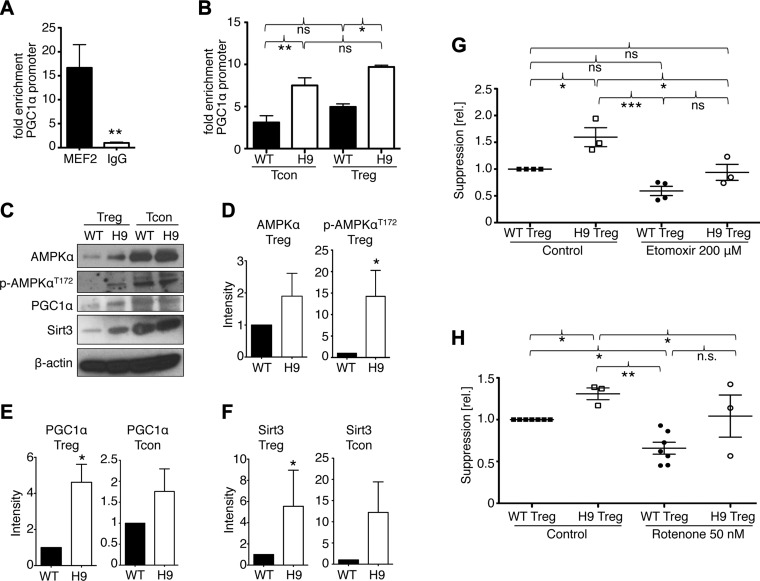
Hdac9 deletion leads to increased Mef2 binding and expression of OXPHOS regulators. A) Chromatin immunoprecipitation assay of genomic DNA from Tcon, stimulated with CD3ε/CD28 mAb for 24 hours, showing Mef2 binding to the Pgc1α promoter. B) Similar to A, comparing WT and Hdac9−/− T cells. C) Western blot analysis of WT and Hdac9−/− Treg and Tcon cells, comparing AMPKα, Pgc1α, and Sirt3. D) Densitometry showing increased AMPKα protein and threonine 172 phosphorylation in Hdac9−/− Tregs. Data pooled from 3 independent experiments. E and F) Densitometry showing Pgc1α (E) and Sirt3 (F) expression is increased in Hdac9−/− Tcon and Treg cells. Data pooled from 4 to 6 independent experiments; *P < 0.05. G and H) Pooled data from 3 to 6 independent Treg suppression assays normalized to vehicle control–treated WT Treg. Hdac9−/− Tregs show superior suppressive function compared to WT cells. Inhibition of β-oxidation (G) or complex I of the mitochondrial respiratory chain (H) diminished but did not abolish the advantage of Hdac9−/− Treg suppressive function. *P < 0.05, **P < 0.01, ***P < 0.001.