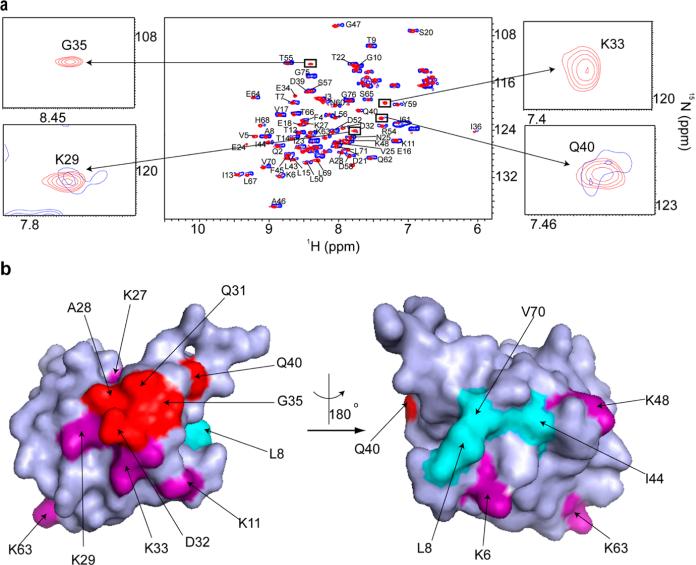
Figure 7.
Ubiquitin quinary interactions block polyubiquitination sites, K27, K29, and K33. (a) Overlay of the in-cell 1H–15N CRINEPT–HMQC–TROSY spectrum of purified REDPRO-labeled ubiquitin transfected into HeLa cells (blue) and the 1H–15N HSQC spectrum of the cell lysate (red). NMR peaks corresponding to K29, K33, G35, and Q40 (insets) are broadened in the in-cell spectrum, suggesting that ubiquitin is involved in transient interactions with cellular components of the cytosol. (b) Residues (red), whose NMR peaks are broadened out (Figure 10 of the Supporting Information), form a contiguous interaction surface involved in ubiquitin quinary interactions (PDB entry 1D3Z). The seven lysines of ubiquitin, which are used for ubiquitylation, are colored purple. K27, K29, and K33 are a part of the interaction surface and are affected by the quinary interactions. The canonical I44 hydrophobic patch of ubiquitin (cyan) spanning L8, I44, and V70 is unperturbed by quinary interactions. The multiplet structure of the in-cell ubiquitin CRINEPT peaks suggests that there is a large population of free ubiquitin, which is in intermediate exchange on the NMR time scale with bound ubiquitin, in HeLa cells.