Figure 1. Effects of Exofucosylation (FTVI treatment) on E-selectin ligand expression by mouse MSC.
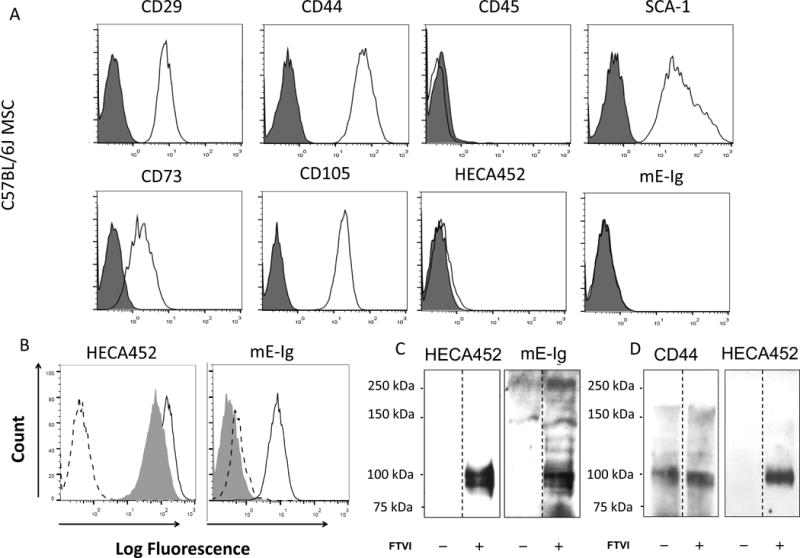
(A) MSC derived from C57BL/6 marrow lacked expression of CD45 and expressed characteristic mouse MSC markers Sca-1, CD29, CD44, CD73 and CD105. Cells lacked reactivity with mAb HECA452 and with E-selectin-Ig chimera (mE-Ig) (istoype= red color and antibody = blue color). (B) FTVI-modified MSC (solid line) stained positive for mAbs HECA452 and were reactive with mE-Ig. Digestion of FTVI-modified MSC with bromelain and proteinase K (shaded histogram) significantly reduced mE-Ig reactivity, but not HECA452 staining, indicating that protease-sensitive glycoproteins serve as the principal E-selectin ligand(s). Dashed line represents staining controls (isotype control for HECA452 staining and calcium chelation with EDTA for mE-Ig staining). (C) Western blot analysis of HECA452 (left) and mE-Ig (right) reactivity of cell lysates of unmodified MSC (−) and FTVI-modified MSC (+). FTVI modification induced HECA452- and mE-Ig-reactive moieties predominantly on a doublet glycoprotein band of ~100 kDa. (D) CD44 was immunoprecipitated from equivalent amounts of cell lysate from FTVI-modified (+) or unmodified (−) MSCs. Immunoprecipitates were then electrophoresed and blotted with anti-CD44 mAbs (KM114 and IM7; left) and with mAb HECA452 (right).
