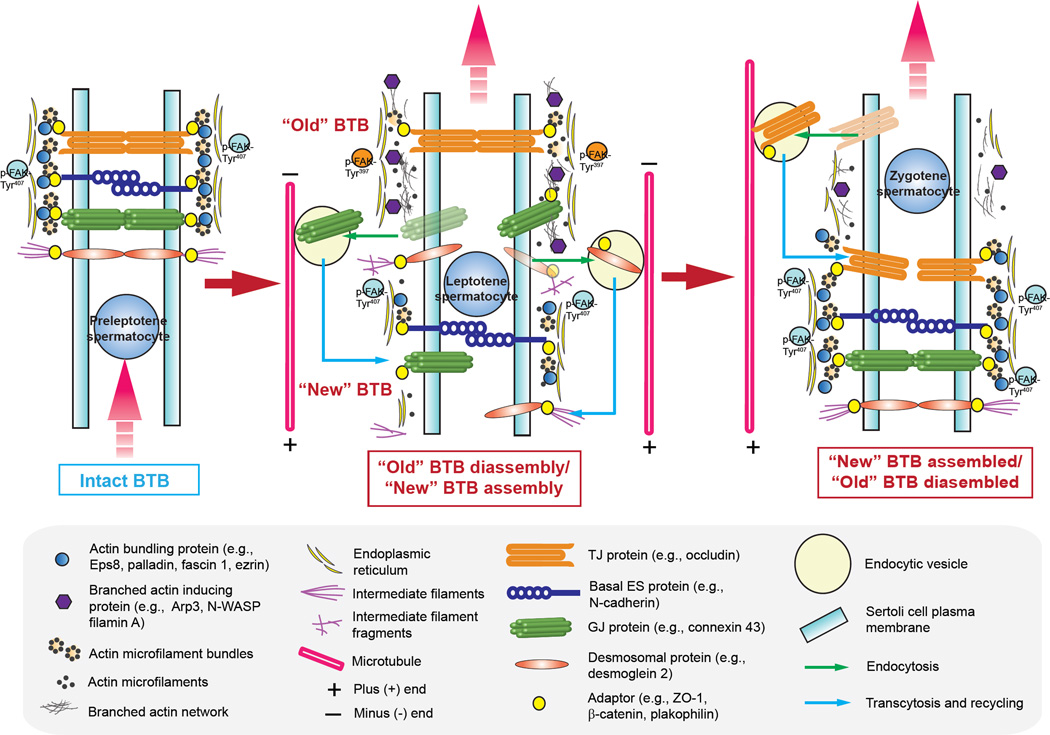
Figure 2. A hypothetical model illustrating the role of actin binding proteins (ABPs) on the transport of preleptotene spermatocytes across the BTB in the mammalian testis during the epithelial cycle of spermatogenesis.
The BTB in the mammalian testis (see left panel) is constituted by coexisting actin-based tight (TJ), basal ES and gap junction (TJ), as well as the intermediate filament-based desmosome between adjacent Sertoli cells, such as at a stage VII tubule, illustrating an intact BTB. The BTB also segregates the seminiferous epithelium into two functional compartments known as the adluminal and the basal compartment so that meiosis I/II and post-meiotic germ cell development all take place behind the BTB in the adluminal compartment. The integrity of the BTB as noted in a stage VII tubule (left panel) is maintained by adhesion protein complexes between adjacent Sertoli cells, such as integral membrane proteins of the TJ (e.g., occludin), basal ES (e.g., N-cadherin) and GJ (connexin43) which are anchored to the actin microfilament bundles via their corresponding adaptors of ZO-1, β-catenin and plakophilin-2. For desmosome, desmosomal integral membrane protein desmoglein-2 is anchored to the intermediate filament via adaptor desmocollin-2. The integrity of actin microfilament bundles are also maintained by the various actin bundling proteins such as Eps8, palladin, fascin 1, ezrin and others (see text for details) (left panel). Furthermore, it is likely that microfilaments that are maintained in their bundled configuration is also supported by p-FAK-Tyr407 at the BTB. Preleptotene spermatocytes differentiate from type B spermatogonia residing in the basal compartment, however, must be transported across the BTB at stage VIII of the epithelial cycle while developing into leptotene spermatocytes at stage IX of the cycle, so that they can prepare for meiosis I and II (see middle panel). During these stages, “old” BTB located above the preleptotene spermatocytes in transit at the BTB undergo extensive remodeling, most notably endocytic vesicle-mediated protein trafficking in which “old” BTB-associated proteins are being endocytosed, transcytosed and recycled to the basal region of preleptotene spermatocytes to assemble the “new” BTB. These changes are made possible by changes in the spatiotemporal expression of several actin binding proteins, mostly notably the up-regulation of branched actin inducing protein Arp2/3-N-WASP protein complex, concomitant with a down-regulation of actin bundling proteins (e.g., Eps8, palladin, fascin 1, and ezrin). This remodeling of BTB is also supported, at least in part, by p-FAK-Tyr397. The net result of these changes induces debundling and branching of the actin microfilaments, destabilizing adhesion protein complexes at the “old” BTB (see middle panel). The transport of spermatocytes and endocytic vesicle-mediated trafficking are also facilitated by the polarized microtubules which serve as the track for transport (see middle panel). By stage XII of the epithelial cycle, leptotene spermatocytes differentiate into zygotene spermatocytes, residing in the adluminal compartment and prepare for meiosis I/II that take place at stage XIV of the cycle, when a “new” BTB is established behind spermatocytes and the “old” BTB is degenerated (see right panel). Thus, remodeling of the BTB in response to different stages of the epithelial cycle to facilitate the transport of preleptotene spermatocytes across the BTB can be effectively regulated by changes in the spatiotemporal expression of the two classes of actin binding proteins, namely actin bundling and branched actin-inducing proteins as depicted herein (see text for details).