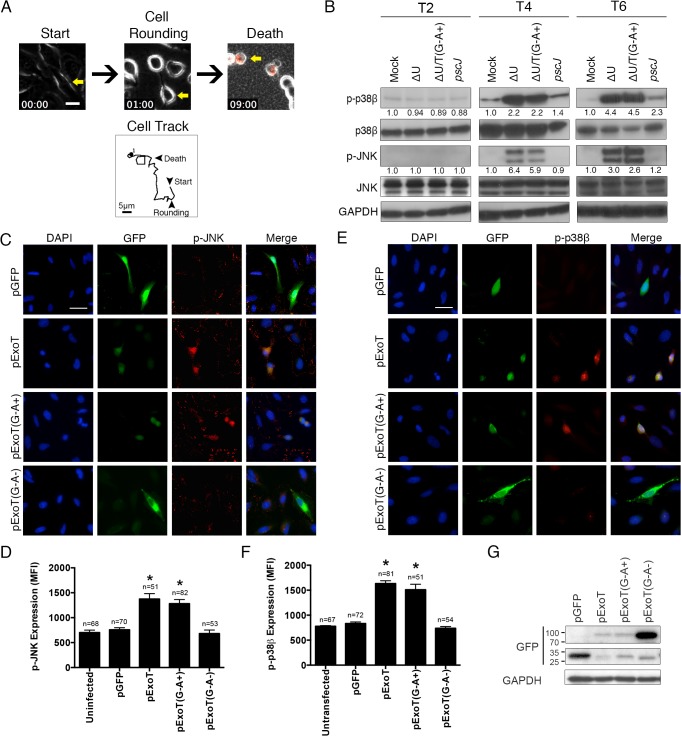
Fig 1. ExoT/ADPRT-intoxicated HeLa cells exhibit anoikis markers.
(A) HeLa cells were infected with PA103∆exoU (∆U) at MOI ~10. Video images were captured every 15 min at 200× magnification. Video frames show a representative ExoT-intoxicated cell (yellow arrow) undergoing cell rounding at 1h and death, as identified by uptake of propidium iodide (red) at 9h. Cell tracking performed by ImageJ, shows this cell movement just prior to cell rounding up until cell death. (B) HeLa cells were treated with PBS (Mock) or infected with PA103∆exoU (∆U); PA103∆exoU/exoT(R149K) (∆U/T(G-A+)); or the T3SS mutant PA103 pscJ::Tn5 (pscJ) at MOI~10. At indicated time points after infection, cell lysates were probed for phosphorylated/activated forms of p38β or JNK (p-p38β or p-JNK) by Western blotting. The fold changes in expression, as compared to mock, are shown underneath. The data were normalized to GAPDH, which was used as loading control. (C and E) HeLa cells were transfected with expression vectors harboring wild type ExoT (pExoT), ExoT with functional ADPRT domain (pExoT(G-A+)), inactive ExoT (pExoT(G-A-)), or empty vector (pGFP). ~24 hr after transfection, cells were fixed and analyzed for either phospho-JNK (p-JNK) (C) or phospho-p38β (E) by IF microscopy. Representative images are shown in (C and E) and the respective expression levels were determined by densitometry and are shown as the mean fluorescent intensity (MFI) ± SEM in (D and F) (* Signifies p<0.001. One-way ANOVA compared with pGFP. Scale bar = 25μm). These data indicate that ExoT/ADPRT is both necessary and sufficient to activate JNK and p38β. (G) HeLa cells transiently transfected as described above and the transient transfection efficiencies were evaluated by Western blotting using anti-GFP antibody. GAPDH was used as a loading control. All data are representative of 3 independent experiments.