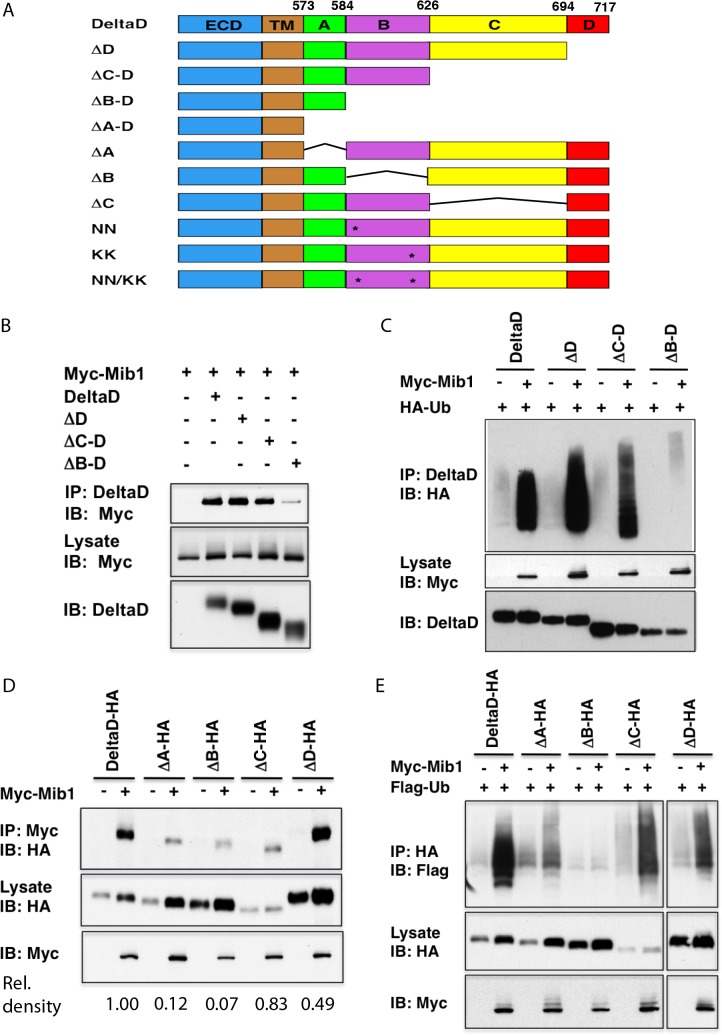
Fig 1. Identification of the Mib1-interacting domain (MID) in the Notch ligand DeltaD.
(A) DeltaD deletion constructs and point mutations. Asterisks represent relative positions of NN and KNxNKK motifs. (B) Mib1 does not interact effectively with DeltaD ∆B-D. Myc-Mib1 was co-immunoprecipitated with full-length DeltaD and truncation mutants (∆D, ∆C-D, ∆B-D) using zdd2 antibody (Ab) and detected with anti-Myc Ab. (C) ∆B-D is not effectively ubiquitylated by Mib1. Full-length and DeltaD truncation mutants, co-transfected with HA-ubiquitin (HA-Ub), with and without Myc-Mib1, were immunoprecipitated with zdd2 Ab and immunoblotted with anti-HA Ab to detect ubiquitylated DeltaD. (D) Delta ∆A (∆A) and Delta ∆B (∆B) interact poorly with Mib1. HA-tagged DeltaD and deletion constructs co-transfected with and without Myc-Mib1 are immunoprecipitated with anti-Myc Ab and detected with anti-HA Ab. Relative density of IP anti-Myc band normalized to lysate anti-HA band. (E) ∆B is not effectively ubiquitylated by Mib1. DeltaD-HA and deletion constructs are immunoprecipitated with anti-HA Ab to detect total ubiquitylated DeltaD with and without Myc-Mib1.