Table 1.
FDA-approved and investigational BCR-ABL1 inhibitors targeting the kinase domain or the myristate pocket.
| Inhibitor | Chemical structure | Binding site/ Inhibitor type |
Regulatory status/ approval |
|---|---|---|---|
| imatinib (Gleevec) |
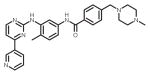
|
ATP-binding site/ATP-competitive | FDA approved/Frontline therapy |
| nilotinib (Tasigna) |
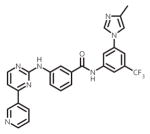
|
ATP-binding site/ATP-competitive | FDA approved/Frontline therapy |
| dasatinib (Sprycel) |
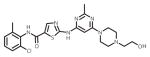
|
ATP-binding site/ATP-competitive | FDA approved/Frontline therapy |
| bosutinib (Bosulif) |
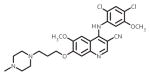
|
ATP-binding site/ATP-competitive | FDA approved/2nd-line therapy |
| ponatinib (Iclusig) |
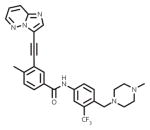
|
ATP-binding site/ATP-competitive | FDA approved/2nd-line therapy |
| ABL001 | (Currently proprietary) | Myristate pocket/Allosteric | Phase I/2nd-line therapy |
