Abstract
Repeated stress can trigger episodes of depression, along with symptoms of anhedonia and anxiety. Although often modeled separately, anxiogenic factors potently modulate hedonic, or appetitive, behavior. While repeated stress can increase anxiety and decrease appetitive behavior, it is not clear whether repeated stress can influence the impact of anxiogenic factors on appetitive behavior. This study tests whether repeated stress shifts behavior in a task that measures anxiogenic-appetitive balance. To test this, adult male rats were trained to lever press for sucrose pellet reward, and the effect of anxiogenic bright light on this behavior was measured. The impact of the bright light anxiogenic stimulus on lever pressing was compared between groups exposed to either daily repeated social defeat stress or control handling. We found that repeated stress reduced exploration in the open field and decreased social interaction, but had minimal effect on baseline lever pressing for reward. Repeated stress substantially enhanced the effect of anxiogenic bright light on lever pressing. This effect was greater two days after the last stress exposure, and began to diminish within two weeks. These data demonstrate that the anxiogenic and anhedonic features induced by repeated stress can be separately measured, and that the impact of anxiogenic stimuli can be greatly enhanced after repeated stress, even in the face of appetitive drive. The data also demonstrate that some apparent anhedonic-like effects of repeated stress can be due to increased sensitivity to anxiogenic stimuli, and may reflect an imbalance in an appetitive approach-withdrawal continuum.
Keywords: social defeat, repeated stress, sucrose, appetitive, anxiety, light
1. Introduction
Two major symptoms of depression are anhedonia and anxiety. These symptoms are sometimes viewed as two components of a tripartite model of depression [1] or expressions of abnormalities along an appetitive approach-withdrawal continuum [2] that has become unbalanced [3]. However, anhedonic and anxiety symptom expression is variable across patients with major depression. Understanding the balance between anxiogenic and appetitive stimuli, and their imbalance in depression, may provide hints about the source of symptom variability in patients, and ways to selectively target depressive symptoms. A balance between approach and withdrawal can be modeled in studies that pit anxiogenic stimuli against appetitive stimuli, and often demonstrate that anxiogenic stimuli can suppress appetitive behaviors. This is commonly observed in tests of novelty-suppressed feeding and drinking [4], conditioned suppression [5] and a range of conflict tests [6, 7].
Repeated stress is a common trigger for depression. Repeated stress can induce symptoms of anxiety and anhedonia in humans [8–12] and in rodent models [13–17]. Stress may cause an imbalance between the response to appetitive and anxiogenic stimuli that is similar to depression. While much is known about how anxiety influences appetitive behavior, little is known about whether stress shifts this balance. Previous studies demonstrate that stress further suppresses drinking in a punished drinking Vogel conflict test [18], and further suppresses feeding in a novel environment [19–21], consistent with a shift in favor of anxiety. However, in previous studies a confounding deprivation state is often imposed on the rat to induce consummatory behavior. In addition, both the appetitive and anxiogenic components are sensitive to stress in those tasks, making it difficult to parse the influence of stress on anxiety and appetitive drive. This study will test whether repeated stress shifts the balance towards anxiety-like behavior when appetitive and anxiogenic conditions are overlaid, using an operant appetitive task that is less sensitive to the effects of acute or repeated stress (leverpressing for sucrose; [22–25]), and does not rely on induction of a deprivation state.
In these experiments, the effects of repeated social defeat on the balance between anxiety-like and appetitive behavior was measured. Bright light is an unconditioned anxiogenic stimulus [26–31] that can suppress appetitive behavior [32]. The interaction between anxiety and appetitive behavior was measured as the effects of bright light on conditioned operant lever pressing for a sucrose pellet. This was compared between adult rats that underwent repeated social defeat or control handling.
2. Materials and Methods
All studies had prior approval of the Rosalind Franklin University Institutional Animal Care and Use Committee, and complied with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Care was taken to minimize animal distress and reduce the number of animals used.
2.1 Animals
Male Sprague-Dawley rats (Harlan Laboratories, Madison, Wisconsin) were used for these studies. Rats arrived at the Rosalind Franklin University vivarium at 53–58 days postnatal. The rats were provided water and food (Rodent Diet 2020× pelleted feed, Harlan Teklad) ad libitum. The housing rooms were set to a 12 h/12 h reverse light–dark cycle. Temperature was maintained between 64 and 79 F and the humidity was maintained between 30 and 70%. Rats were housed 2–3 per cage (polycarbonate solid bottom, 43.2 × 21.5 × 20.3 height, in cm), and were habituated to the facility for 1–2 weeks. Rats were handled daily for three days prior to initiation of training. All experiments were performed during the dark phase of the light-dark cycle.
2.2 Appetitive Conditioning
2.2.1 Apparatus
Conditioning was performed in an operant chamber (Med Associates, ENV-001, St. Albans, VT). The chamber was enclosed within a sound-attenuating cabinet (Med Associates). Each cabinet was affixed with an IR-sensitive digital camera (Fire-i, Unibrain, San Ramon, CA), infrared lighting, and dim white house lighting (20 lux). There was a fan in each cabinet that provided airflow and ~60 dB of ambient noise. The chamber was also fitted with 2 levers, one designated as the active lever and the other designated as the inactive lever, a cue light placed proximal to the levers, and a food receptacle where sucrose pellets were delivered.
2.2.2 Appetitive Conditioning Procedure
Rats were taken from their home cages and individually transferred by transport cages to the procedure room, where rats were placed in the operant chamber. After each session, rats were returned to their home cage. Each rat was habituated to the operant chamber for 2 sessions (one/day) before training. During these 2 sessions, sucrose pellets (45 mg) were placed in both the food receptacle and on the active lever to facilitate subsequent learning. After two habituation sessions, appetitive conditioning sessions began. Each appetitive conditioning session was 30 minutes in length. Sucrose pellets were remotely delivered when the rat neared the active lever, in order to facilitate differentiation between the active and inactive lever. When the rat pressed the active lever, the cue light (green, 2.5 cm diameter, 1 sec) was triggered immediately and a sucrose pellet was delivered 1 second after the cue light (Fixed ratio schedule (FR) 1). Manual delivery of pellets ceased by the third training session, as rats displayed preference for the active levers by this session. By the fourth day of training, and throughout the remainder of the entire experiment, rats consumed all delivered pellets. The number of presses on active and inactive levers was recorded for each session. All rats were required to meet a minimum criterion of 35 active lever presses by their final session in order to move onto the next phase of the experiment. This criterion was attained between 7 to 9 days. One day after criteria was reached, rats were tested at a FR 4 schedule for 30 minutes.
2.3 Treatment groups: Social defeat and control
There were two treatment groups: social defeat stress and control. These groups were further divided into two subgroups: rats tested after 2 days (n=8 rats/group, and a second cohort of n=8 rats/group for experiments with no anxiogenic light; see below, 2.6 Suppression of Appetitive Behavior by Anxiety) and rats tested after 2 weeks (14–17 days; n=14 rats/group) for a total of 60 rats. This does not include 3 rats that were excluded because they did not reach lever pressing criteria. The number of rats was based on the expected effect size from other studies. Rats were matched for number of lever presses on the day that criteria was reached, and then randomly assigned into control or social defeat treatment group. Beginning one day after rats were tested at the FR 4 schedule, the rats were exposed to social defeat or control handling once/day for 5 consecutive days (Fig 1A). Social defeat began by transporting rats to a procedure room, and placing an “intruder” (male Sprague-Dawley rat) into the home cage of a “resident” (male retired breeder Long Evans rat, Harlan Laboratories). The two rats were allowed to be in physical contact with each other for a maximum period of 15 minutes. They were separated when one of the following conditions was met: submission of the intruder, 15 minutes with no submission, 10 attacks with no submission, 5 minutes without any attack, or any attack that wounded a rat. The intruder rat was separated using a wire mesh cage placed over the animal and it remained in the residents’ cage for an additional 15 minutes to permit unrestricted visual, auditory, and olfactory contact without any further physical attacks. Intruder rats were rotated through different resident rats each day. Control handling comprised of placement of rats into a transport cage for 20 minutes. At the end of each session, rats were returned to their home cage. Experimental rats remained in their home cage for 2 days or 14–17 days before subsequent behavioral testing. After this interval, rats were tested in the open field, social interaction, operant lever pressing for sucrose pellets, and suppression of sucrose seeking by anxiogenic stimulus (Fig 1A). Over the course of these experiments, rats were weighed daily and their body condition was assessed.
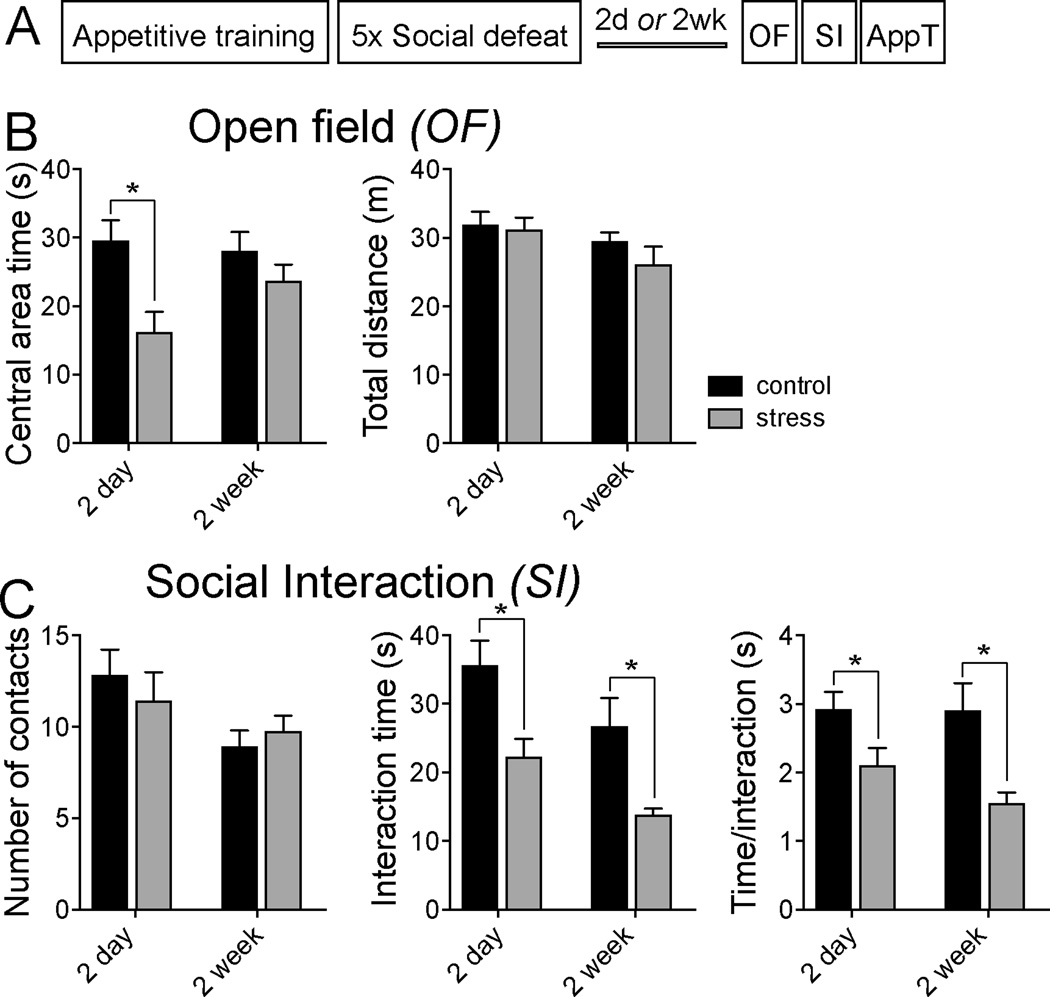
Figure 1. Repeated social defeat increases anxiety-like behavior.
A) Schematic of the experimental timeline. One day after completion of appetitive training, rats were subjected to daily social defeat or control procedures for 5 consecutive days. Beginning after 2 days or 2 weeks, rat behavior was measured in the open field test (OF), social interaction test (SI), and appetitive test (AppT). B) Rats that were exposed to social defeat displayed decreased exploration in the center of the open field when measured at 2 days but not 2 weeks (left), with no significant difference in total distance traveled (right). C) While there was no significant effect of social defeat on the number of social interactions (left), there was a significant decrease in the total time of social interaction (middle) and the duration of social contacts (right). * p<0.05 in post hoc Holm-Sidak after two-way ANOVA.
2.4 Open Field
Rats were individually placed in an open field (61 × 89 cm) in a room with dim white light (20–25 lux; 5 min) and dim red light. Video was captured with an IR-sensitive camera (Fire-I, Unibrain). The field was divided into 16 boxes (15.2×14.8 cm) during analysis. The central area was defined as the middle four boxes. Exploration in the open field was quantified as the amount of time the rat was in the central area of the field (AnyMaze software, Stoelting Co., Wood Dale, IL).
2.5 Social Interaction
One day after the open field test, a novel rat was placed in the open field, and the test rat was placed in the same open field (5 minutes, same conditions as above). The novel rats had a body weight within 50 g of the test rats, and had previously been exposed to this open field for at least 10 minutes. As above, video was captured with an IRsensitive camera (Fire-I, Unibrain). The video was used by trained raters to measure the number of rat interactions and the total amount of time in contact. The raters showed >85% concordance in their tabulations before data was compiled. The trained rater manually tabulated the number of times the test rat approached and interacted with the other rat (defined as exploration of novel rat with nose) during video replay, and used a digital stopwatch to quantify the total time of interaction during a separate video replay.
2.6 Suppression of Appetitive Behavior by Anxiety
Rats were individually placed in the operant chamber for a period of 15 minutes, divided into three 5-minute phases. Rats could lever press freely to obtain sucrose pellets (FR 4) during all three phases. During the first 5-minute phase (OFF1), lighting conditions were the same as light during training (20 lux). During the second 5-minute phase (ON), an anxiogenic bright light (200 lux) was illuminated in the cabinet. For the final 5-minute phase (OFF2), the bright light was turned off and normal light conditions (20 lux) were restored. Active and inactive lever presses were recorded for each 5-minute session. The raw number of lever presses were used for analysis of the effects of light or stress. To compare effects of light and stress between the 2 day and 2 week delay testing conditions, the effect of light on the number of lever presses in control and stress groups was compared: [(OFF1- ON) + (OFF1 - (OFF2)]/2. In a separate group of rats, the light remained off during all three phases of the session (N=8 rats/group).
2.7 Data Acquisition and Analysis
All data was video recorded using AnyMaze software. Detection thresholds for rat tracking were set based upon >95% convergence with a manual rater. Data analysis was performed using GraphPad Prism software (La Jolla, CA). Significance was set at p < 0.05. Data was tested for homogeneity of variance (Bartlett’s test) and distribution normality (Kolmgorov and Smirnov test). If data passed these tests, parametric statistical approaches were used. When two factors were compared, a two-way ANOVA was used. Significant main effects and interactions were followed by post hoc Holm- Sidak tests, when appropriate. For one factor planned comparisons, two-tailed unpaired t-tests were used to compare groups. Raw values were used for most analyses. Where noted, the effect size was compared (defined as [Xstress – X̅control], where Xstress = the values from individual rats from the stress group, and X̅control = the average value of the control group).
3. Results
3.1 Social defeat effectively produces anxiety-like behavior
Separate groups of rats were tested 2 days or 2 weeks after the final stress or control session. Similar to previous studies, repeated social defeat reduced open field exploration (Fig 1B; time in center area; two-way ANOVA, main effect of stress p=0.006, F(1,40)=8.473) when tested 2 days (n=8 rats/group, p<0.05, post hoc Holm-Sidak) but not 2 weeks after the final stress (n=14 rats/group; p>0.05, post hoc Holm-Sidak). There was no effect of stress on total distance traveled (Fig 1B; two-way ANOVA, main effect of stress p=0.380, F(1,40)=0.788). Social defeat also reduced social interaction time (Fig 1C; two-way ANOVA, main effect of stress p=0.0004, F(1,40)=14.900) when tested 2 days (n=8/group; p<0.05, post hoc Holm-Sidak) or 2 weeks after the final stress (n=14/group; p<0.05, post hoc Holm-Sidak). Repeated stress did not significantly decrease the total number of contacts (Fig 1C; two-way ANOVA, main effect of stress p=0.821, F(1,40)=0.005), although there was a significant effect of stress on the duration of each social contact (Fig 1C; two-way ANOVA, main effect of stress p=0.002, F(1,40)=10.870). These data are consistent with increased anxiety-like behavior after social defeat, and a diminishment of the effects of social defeat stress on open field exploration over 2 weeks [effect of stress on center time, 2 day (−13.5 ± 3.2 s) compared to 2 week (−4.4 ± 2.6 s), two-tailed unpaired t-test, p=0.040, t=2.201, df=20], but persistent effects of stress on social interaction over two weeks [effect of stress on time of interaction, 2 day (−13.4 ± 2.8 s) compared to 2 week (−12.9 ± 1.09 s), two-tailed unpaired t-test, p=0.860, t=0.178, df=20]. In addition, these data provide confirmatory evidence for effectiveness of this stress model.
3.2 Anxiogenic light decreases active lever presses in socially defeated animals
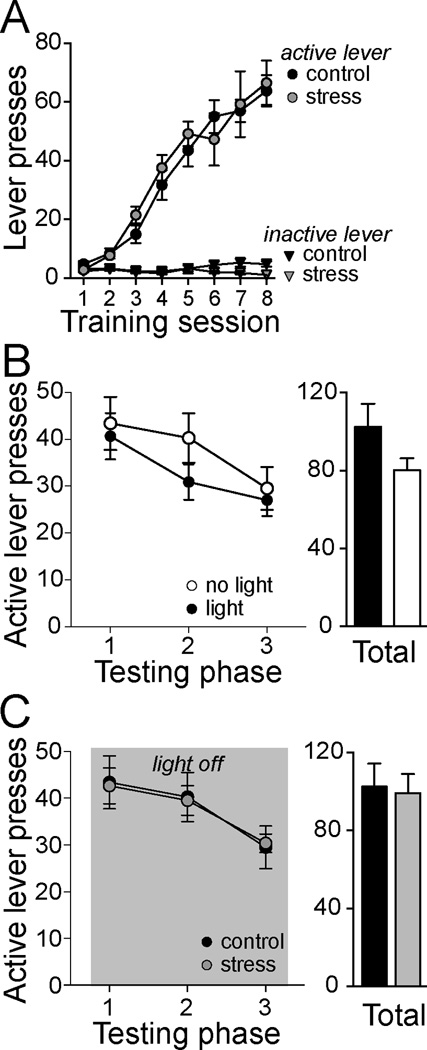
Only rats that displayed acquisition of lever pressing for sucrose to criteria were included in analysis. Rats were matched for number of lever presses on the day that criteria was reached, and then randomly assigned into control or stress treatment group. This matching was effective, as there was no significant difference between groups on active lever presses (Fig 2A; two-way ANOVA, main effect of group p=0.569, F(7,381)=0.326) or inactive lever presses (Fig 2A; two-way ANOVA, main effect of group p=0.232, F(1,381)=1.327) across acquisition. Rats displayed a significant increase of active lever-pressing across training (two-way ANOVA, main effect of session p<0.0001, F(1,381)=46.210), reflective of acquisition of this operant behavior.
Figure 2. Repeated social defeat does not impact appetitive lever-pressing.
A) Rats were trained in an appetitive conditioning task to lever press for sucrose, were matched for lever-pressing and then randomly assigned to control or stress groups. Indicative of the effectiveness of the matching, there was no significant difference between these groups in the number of active or inactive lever presses before control or stress procedures. B) Presentation of a bright light suppressed lever-pressing in the control group, compared to the no-light condition. The total number of active lever presses during the three 5-minute segments of appetitive testing was not significantly different (right). C) In a separate group of rats, there was no significant difference in lever-pressing between control and stress groups on the number of active lever presses in 5-minute segments (Testing phase) when measured after control or stress procedures (left). There was no significant difference between control and stress groups in the total number of active lever presses of the three 5-minute segments of appetitive testing (right).
Two days
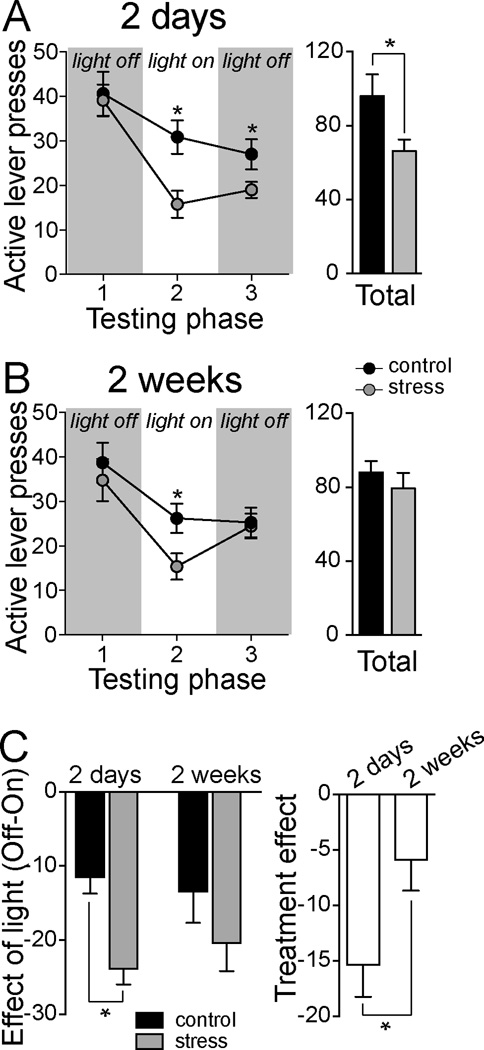
When tested at 2 days after the final stress or control session, significant differences in lever-pressing behavior emerged. Anxiogenic light significantly reduced lever pressing compared to the no-light condition (Fig 2B; two-way RMANOVA, light × phase interaction p=0.048 F(2,28)=3.391). This verifies the effectiveness of bright light in reducing appetitive behavior under these conditions. This effect of light was evident in control and stress groups (Fig 3A, left; two-way repeated measures ANOVA, main effect of light p<0.0001, F(2,28)=81.100). However, there was a significant effect of stress that depended on the phase of testing (two-way repeated measures ANOVA, stress × phase interaction p=0.0005, F(2,28)=10.100). There was no effect of stress on lever pressing during the initial lights-off phase (p>0.05, post hoc Holm-Sidak, control compared to stress during first 5 minutes (OFF1)), however, the effect of bright light (during lights-on phase (ON)) was significantly greater in social defeat rats (p<0.05, post hoc Holm-Sidak, control compared to stress during second 5 minutes). Furthermore, the bright light caused longer-lasting effects in the stress group, so that even when the light was turned back off, lever pressing did not return to the same level as control rats (Fig 3A, left; p<0.05, post hoc Holm-Sidak, control compared to stress during third 5 minutes (OFF2)). This led to a significant difference in the total number of sucrose pellets delivered during the session (Fig 3A, right; two-tailed unpaired t-test, p=0.043, t=2.226, df=14). This difference is not simply due to differences in satiety, as stress did not influence lever pressing when comparing equivalent 5-minute phases with no bright light in a different group of rats subjected to the same experimental design (Fig 2C, left; two-way repeated measures ANOVA, main effect of stress p=0.837, F(1,14)=0.044, n=8 rats/group), and there was no significant effect of stress on the total number of sucrose pellets delivered during the testing session (Fig 2C, right; two-tailed unpaired t-test, p=0.843, t=0.202, df=14).
Figure 3. Repeated social defeat increases the effect of anxiogenic bright light on appetitive lever-pressing.
Rats were exposed to a testing session with 5-minute offon- off segments of bright light. A) Bright light presented during appetitive testing suppressed lever pressing when tested after 2 days. The effect of bright light was greater in rats that were exposed to repeated stress and this effect lasted longer than the duration of the light (left, *p<0.05 in post hoc Holm-Sidak after two-way ANOVA). This led to a decrease in the total number of active lever presses during this appetitive testing session in rats that were exposed to repeated stress (right). B) Bright light presented during appetitive testing suppressed lever pressing when tested after 2 weeks. The effect of bright light was greater in rats that were exposed to repeated stress, but did not last longer than the duration of the light (left, *p<0.05 in post hoc Holm-Sidak after two-way ANOVA). There was no significant difference in the total number of active lever presses during this appetitive testing session between control and stress groups (right). C) To directly compare the effects of stress when tested after 2 days or 2 weeks, the effect of bright light was subtracted from baseline (see Methods, 2.7 Data Acquisition and Analysis) and compared across time. Stress significantly enhanced the effect of anxiogenic light on active lever pressing (left), and this effect was greater when measured after 2 days compared to 2 weeks.
Two weeks
When tested at two weeks after the final stress or control session in a separate group of rats, bright light still significantly decreased lever pressing (Fig 3B; two-way repeated measures ANOVA, main effect of light p<0.0001, F(2,52)=19.500). Furthermore, the effect of bright light was significantly greater in social defeat rats (p<0.05, post hoc Holm-Sidak, control compared to stress during second 5 minutes (ON)). However, when the light was turned back off, the stress group recovered to lever-pressing levels equivalent to controls (Fig 3B, left; p<0.05, post hoc Holm-Sidak, control compared to stress during third 5 minutes (OFF2)), and stress did not lead to a significant difference in the total number of sucrose pellets delivered during the session (Fig 3B, right; two-tailed unpaired t-test, p=0.407, t=0.842, df=26). Furthermore, when comparing the effect of bright light on lever pressing between 2 days and 2 weeks, there was a main effect of stress (Fig 3C, left; two-way ANOVA, main effect of stress p=0.009, F(1,40)=7.46, stress × time interaction p=0.042, F(1,40)=4.417). Stress had a greater impact when measured after 2 days compared to 2 weeks (Fig 3C, right; two-tailed unpaired t-test, p=0.039, t=2.211, df=20).
4. Discussion
The present study demonstrated that repeated social defeat stress shifted the balance between approach and withdrawal behavior. Anxiogenic bright light suppressed leverpressing for a reward. Stress enhanced this suppressive effect of anxiogenic bright light. This effect of stress was not explained by reduced basal reward seeking behavior, because stress had minimal effect on lever pressing to obtain a reward when the bright light was not present. Instead, the effects of stress are most likely due to enhanced impact of anxiogenic stimuli on reward-seeking. In support of this, repeated social defeat stress was anxiogenic, decreasing exploration in the open field and decreasing social interaction. In addition, the effects of stress on the interaction between appetitive and anxiogenic stimuli lasted at least 2 weeks after the final stress exposure, mirroring some of the enhanced anxiety behavior in the social interaction test.
Previous studies demonstrated that repeated stress suppresses unconditioned approach of appetitive stimuli, such as food and sucrose. This is often interpreted as anhedonia-like behavior. However, in many studies, a confounding deprivation state is introduced by food or water restriction. Furthermore, in many studies that measure appetitive approach, there is a component of anxiety-like behavior that must be overcome by the rodent [33–38]. This anxiety component is sensitive to stress [39–45] and may produce apparent decreased reward seeking by increasing anxiety-like withdrawal behavior. However, stress has less impact on simple conditioned appetitive behaviors [22–25], as further affirmed in the current study. Because this conditioned appetitive behavior is not strongly impacted by stress, it provides an opportunity to measure the effect of stress on the interaction between anxiogenic and appetitive stimuli. The appetitive component (lever pressing) was separately measureable, not significantly altered by stress, and sensitive to anxiogenic stimuli. We found that this sensitivity to anxiogenic bright light was selectively enhanced by repeated stress.
In the current study, repeated stress had minimal influence on basic characteristics of lever-pressing for sucrose pellets, consistent with previous studies [22, 23, 46] (but see [47]). This could be interpreted as minimal effect of stress on reward processing per se. Repeated stress often shifts choice in sucrose preference test and consumption of appetitive foods under certain situations, such as limited access to the palatable substance [48–51]. However, neither acute nor repeated stress strongly influences consumption of freely available palatable food, with some evidence of repeated stress increasing consumption [52–54]. One possible interpretation is that stress has a more selective suppressive effect on appetitive behavior when there are conflicting or opposing components. In fact, acute stress can reduce lever pressing for reward in such circumstances. For instance, acute stress reduces preference for larger yet costlier rewards [24], and reduces reward-related responding if the effort requirement is very demanding (e.g. >FR50; [55]). Stress can also enhance the motivational effects of downward shifts in reward magnitude on reward-seeking [56] and the effects of reward devaluation via satiety manipulations. [57]. These effects of stress appear to modify the perception of the value of an appetitive stimulus, particularly when there is some change in its relative value. This may be reflected by negative cognitive bias in patients with depression [58–60]. Similarly, an anxiogenic stimulus can devalue a co-occurring appetitive stimulus, and increased anxiogenicity after stress may further devalue the appetitive stimulus. In several animal studies where anxiogenic, appetitive, or ambiguous stimuli are presented after repeated stress, animals tend to make choices guided by avoidance of anxiogenic stimuli instead of appetitive stimuli [61–63] or "pessimistic" choice of a lesser reward [64, 65].
Activation of the ventral striatum is closely associated with reward processing in humans and rodents, while activation of the amygdala and extended amygdala is associated with anxiety. A wide range of neural structures is sensitive to acute and repeated stress. Acute stress or repeated stress can lead to reduced ventral striatal processing of reward and reward-related cues in humans [66–72]. Similarly, depression is also associated with reduced activation of the ventral striatum in response to rewards and cues associated with rewards [68, 73]. Aversive stimuli reduce the response of striatal regions to appetitive stimuli [74]. On the other hand, acute aversive or anxiogenic stimuli recruit the amygdala and extended amygdala [75–79]. Extended amygdala circuitry may be recruited into modifying behavior more readily when a prolonged anxiogenic state is induced [80, 81], after a history of stress exposure [81–89], and in patients with depression [90–93]. The increased activity of the amygdala and associated increases in anxiety may, in turn, modify activity of the ventral striatum and appetitive behavior. In rodents, the impact of nucleus accumbens manipulation on appetitive function can be strongly modified by anxiogenic environments [94]. Lever pressing for cued delivery of a reward relies on a pathway from the basolateral amygdala to the ventral striatum [95, 96], and anxiogenic influences of bright light are mediated by the bed nucleus of the stria terminalis (BNST) portion of the extended amygdala [97]. There is evidence for a direct pathway from the BNST to the ventral striatum [98–101], and ventral tegmental area [102, 103]. In addition, the BNST can modulate NAc-mediated motivated behaviors [104–109]. Thus, increased sensitivity of extended amygdala circuitry to anxiogenic stimuli that occurs following repeated stress may lead to a greater ability of this system to suppress ongoing reward seeking behaviors that are mediated, in part, by the NAc. As such, the shift in reward-seeking behavior observed after repeated stress in the current study may reflect imbalance in the recruitment of the NAc and amygdala during these behaviors.
The expression of anxiety and anhedonic symptoms varies across patients with major depression, and can vary across time. An understanding of the factors that differentially impact these symptoms, and how they interact, may lead to insight into factors that contribute to symptom variability. In addition, better understanding of these factors may aid in the development of more selective therapeutics to target different depressive symptomology.
Highlights.
Anxiogenic bright light decreases lever-pressing for reward
Stress increases anxiety-like behavior
Stress increases the effect of anxiogenic light on lever-pressing for reward
Acknowledgements
The authors thank Dr. Stan Floresco for very helpful comments and suggestions and Dr. Robert Twining for helpful advice. Grant support provided by National Institutes of Health (MH084970). The funding providers had no role in study design, collection, analysis and interpretation of data, writing of the report or in the decision to submit this article for publication.
Abbreviations
- ANOVA
analysis of variance
- FR
fixed ratio
- OF
open field
- SI
social interaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 2.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 3.Mendl M, Burman OH, Paul ES. An integrative and functional framework for the study of animal emotion and mood. Proc Biol Sci. 2010;277:2895–2904. doi: 10.1098/rspb.2010.0303. 10.1098/rspb.2010.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stout JC, Weiss JM. An animal model for measuring behavioral responses to anxiogenic and anxiolytic manipulations. Pharmacol Biochem Behav. 1994;47:459–465. doi: 10.1016/0091-3057(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 5.Desiderato O, Newman A. Conditioned suppression produced in rats by tones paired with escapable or inescapable shock. J Comp Physiol Psychol. 1971;77:427–431. doi: 10.1037/h0031880. [DOI] [PubMed] [Google Scholar]
- 6.Bond NW, Blackman DE, Scruton P. Suppression of operant behavior and schedule-induced licking in rats. J Exp Anal Behav. 1973;20:375–383. doi: 10.1901/jeab.1973.20-375. 10.1901/jeab.1973.20-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 8.Hovens JG, Wiersma JE, Giltay EJ, van Oppen P, Spinhoven P, Penninx BW, et al. Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatr Scand. 2010;122:66–74. doi: 10.1111/j.1600-0447.2009.01491.x. 10.1111/j.1600-0447.2009.01491.x. [DOI] [PubMed] [Google Scholar]
- 9.Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. 2007;164:1521–1529. doi: 10.1176/appi.ajp.2007.06091564. quiz 1622. 10.1176/appi.ajp.2007.06091564. [DOI] [PubMed] [Google Scholar]
- 10.Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- 11.Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- 12.van Veen T, Wardenaar KJ, Carlier IV, Spinhoven P, Penninx BW, Zitman FG. Are childhood and adult life adversities differentially associated with specific symptom dimensions of depression and anxiety? Testing the tripartite model. J Affect Disord. 2013;146:238–245. doi: 10.1016/j.jad.2012.09.011. 10.1016/j.jad.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen CK, Arnt J, Sanchez C. Intracranial self-stimulation and sucrose intake differ as hedonic measures following chronic mild stress: interstrain and interindividual differences. Behav Brain Res. 2000;107:21–33. doi: 10.1016/s0166-4328(99)00110-2. [DOI] [PubMed] [Google Scholar]
- 14.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 15.Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Von Frijtag JC, Van den Bos R, Spruijt BM. Imipramine restores the long-term impairment of appetitive behavior in socially stressed rats. Psychopharmacology (Berl) 2002;162:232–238. doi: 10.1007/s00213-002-1093-3. 10.1007/s00213-002-1093-3. [DOI] [PubMed] [Google Scholar]
- 17.Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- 18.Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, et al. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000;97:12852–12857. doi: 10.1073/pnas.220232997. 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiol Behav. 2011;103:210–216. doi: 10.1016/j.physbeh.2011.02.001. 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Horm Behav. 2008;53:386–394. doi: 10.1016/j.yhbeh.2007.11.001. 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Barr AM, Phillips AG. Chronic mild stress has no effect on responding by rats for sucrose under a progressive ratio schedule. Physiol Behav. 1998;64:591–597. doi: 10.1016/s0031-9384(98)00060-2. [DOI] [PubMed] [Google Scholar]
- 23.Kant GJ, Bauman RA. Effects of chronic stress and time of day on preference for sucrose. Physiol Behav. 1993;54:499–502. doi: 10.1016/0031-9384(93)90242-8. [DOI] [PubMed] [Google Scholar]
- 24.Shafiei N, Gray M, Viau V, Floresco SB. Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology. 2012;37:2194–2209. doi: 10.1038/npp.2012.69. 10.1038/npp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widman DR, Abrahamsen GC, Rosellini RA. Environmental enrichment: the influences of restricted daily exposure and subsequent exposure to uncontrollable stress. Physiol Behav. 1992;51:309–318. doi: 10.1016/0031-9384(92)90146-s. [DOI] [PubMed] [Google Scholar]
- 26.Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 27.DeFries JC, Hegmann JP, Weir MW. Open-field behavior in mice: evidence for a major gene effect mediated by the visual system. Science. 1966;154:1577–1579. doi: 10.1126/science.154.3756.1577. [DOI] [PubMed] [Google Scholar]
- 28.File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia AM, Cardenas FP, Morato S. Effect of different illumination levels on rat behavior in the elevated plus-maze. Physiol Behav. 2005;85:265–270. doi: 10.1016/j.physbeh.2005.04.007. 10.1016/j.physbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Slawecki CJ. Comparison of anxiety-like behavior in adolescent and adult Sprague-Dawley rats. Behav Neurosci. 2005;119:1477–1483. doi: 10.1037/0735-7044.119.6.1477. 10.1037/0735-7044.119.6.1477. [DOI] [PubMed] [Google Scholar]
- 31.Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 32.Barker DJ, Sanabria F, Lasswell A, Thrailkill EA, Pawlak AP, Killeen PR. Brief light as a practical aversive stimulus for the albino rat. Behav Brain Res. 2010;214:402–408. doi: 10.1016/j.bbr.2010.06.020. 10.1016/j.bbr.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 34.Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology (Berl) 1989;97:277–279. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- 35.Britton DR, Britton KT. A sensitive open field measure of anxiolytic drug activity. Pharmacol Biochem Behav. 1981;15:577–582. doi: 10.1016/0091-3057(81)90212-4. [DOI] [PubMed] [Google Scholar]
- 36.Griebel G, Belzung C, Misslin R, Vogel E. The free-exploratory paradigm: an effective method for measuring neophobic behaviour in mice and testing potential neophobia-reducing drugs. Behav Pharmacol. 1993;4:637–644. [PubMed] [Google Scholar]
- 37.Merali Z, Levac C, Anisman H. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry. 2003;54:552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- 38.Shephard RA, Estall LB. Effects of chlordiazepoxide and of valproate on hyponeophagia in rats. Evidence for a mutual antagonism between their anxiolytic properties. Neuropharmacology. 1984;23:677–681. doi: 10.1016/0028-3908(84)90150-3. [DOI] [PubMed] [Google Scholar]
- 39.Burgado J, Harrell CS, Eacret D, Reddy R, Barnum CJ, Tansey MG, et al. Two weeks of predatory stress induces anxiety-like behavior with co-morbid depressive-like behavior in adult male mice. Behav Brain Res. 2014;275:120–125. doi: 10.1016/j.bbr.2014.08.060. 10.1016/j.bbr.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Dev Psychobiol. 2013;55:849–859. doi: 10.1002/dev.21077. 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- 41.Kinn AM, Gronli J, Fiske E, Kuipers S, Ursin R, Murison R, et al. A double exposure to social defeat induces sub-chronic effects on sleep and open field behaviour in rats. Physiol Behav. 2008;95:553–561. doi: 10.1016/j.physbeh.2008.07.031. 10.1016/j.physbeh.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 42.Meerlo P, Overkamp GJ, Daan S, Van Den Hoofdakker RH, Koolhaas JM. Changes in Behaviour and Body Weight Following a Single or Double Social Defeat in Rats. Stress. 1996;1:21–32. doi: 10.3109/10253899609001093. [DOI] [PubMed] [Google Scholar]
- 43.Mikics E, Toth M, Varju P, Gereben B, Liposits Z, Ashaber M, et al. Lasting changes in social behavior and amygdala function following traumatic experience induced by a single series of foot-shocks. Psychoneuroendocrinology. 2008;33:1198–1210. doi: 10.1016/j.psyneuen.2008.06.006. 10.1016/j.psyneuen.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Nam H, Clinton SM, Jackson NL, Kerman IA. Learned helplessness and social avoidance in the Wistar-Kyoto rat. Front Behav Neurosci. 2014;8:109. doi: 10.3389/fnbeh.2014.00109. 10.3389/fnbeh.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez Echandia EL, Gonzalez AS, Cabrera R, Fracchia LN. A further analysis of behavioral and endocrine effects of unpredictable chronic stress. Physiol Behav. 1988;43:789–795. doi: 10.1016/0031-9384(88)90378-2. [DOI] [PubMed] [Google Scholar]
- 46.Anderson SM, Saviolakis GA, Bauman RA, Chu KY, Ghosh S, Kant GJ. Effects of chronic stress on food acquisition, plasma hormones, and the estrous cycle of female rats. Physiol Behav. 1996;60:325–329. doi: 10.1016/0031-9384(96)00023-6. [DOI] [PubMed] [Google Scholar]
- 47.Pielock SM, Braun S, Hauber W. The effects of acute stress on Pavlovian-instrumental transfer in rats. Cogn Affect Behav Neurosci. 2013;13:174–185. doi: 10.3758/s13415-012-0129-3. 10.3758/s13415-012-0129-3. [DOI] [PubMed] [Google Scholar]
- 48.Cheeta S, Broekkamp C, Willner P. Stereospecific reversal of stress-induced anhedonia by mianserin and its (+)-enantiomer. Psychopharmacology (Berl) 1994;116:523–528. doi: 10.1007/BF02247488. [DOI] [PubMed] [Google Scholar]
- 49.Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- 50.Kioukia N, Bekris S, Antoniou K, Papadopoulou-Daifoti Z, Christofidis I. Effects of chronic mild stress (CMS) on thyroid hormone function in two rat strains. Psychoneuroendocrinology. 2000;25:247–257. doi: 10.1016/s0306-4530(99)00051-7. [DOI] [PubMed] [Google Scholar]
- 51.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 52.Ely DR, Dapper V, Marasca J, Correa JB, Gamaro GD, Xavier MH, et al. Effect of restraint stress on feeding behavior of rats. Physiol Behav. 1997;61:395–398. doi: 10.1016/s0031-9384(96)00450-7. [DOI] [PubMed] [Google Scholar]
- 53.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 54.Hancock SD, Menard JL, Olmstead MC. Variations in maternal care influence vulnerability to stress-induced binge eating in female rats. Physiol Behav. 2005;85:430–439. doi: 10.1016/j.physbeh.2005.05.007. 10.1016/j.physbeh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120:842–851. doi: 10.1037/0735-7044.120.4.842. 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowers WJ, Attiast E, Amit Z. Stress enhances the response to reward reduction but not food-motivated responding. Physiol Behav. 1999;67:777–782. doi: 10.1016/s0031-9384(99)00129-8. [DOI] [PubMed] [Google Scholar]
- 57.Ghiglieri O, Gambarana C, Scheggi S, Tagliamonte A, Willner P, De Montis MG. Palatable food induces an appetitive behaviour in satiated rats which can be inhibited by chronic stress. Behav Pharmacol. 1997;8:619–628. doi: 10.1097/00008877-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 58.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 59.Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CAJ, et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- 60.Williams LM, Gatt JM, Schofield PR, Olivieri G, Peduto A, Gordon E. 'Negativity bias' in risk for depression and anxiety: brain-body fear circuitry correlates, 5-HTT-LPR and early life stress. Neuroimage. 2009;47:804–814. doi: 10.1016/j.neuroimage.2009.05.009. 10.1016/j.neuroimage.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Enkel T, Gholizadeh D, von Bohlen Und Halbach O, Sanchis-Segura C, Hurlemann R, Spanagel R, et al. Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology. 2010;35:1008–1015. doi: 10.1038/npp.2009.204. 10.1038/npp.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papciak J, Popik P, Fuchs E, Rygula R. Chronic psychosocial stress makes rats more 'pessimistic' in the ambiguous-cue interpretation paradigm. Behav Brain Res. 2013;256:305–310. doi: 10.1016/j.bbr.2013.08.036. 10.1016/j.bbr.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 63.Paul ES, Harding EJ, Mendl M. Measuring emotional processes in animals: the utility of a cognitive approach. Neurosci Biobehav Rev. 2005;29:469–491. doi: 10.1016/j.neubiorev.2005.01.002. 10.1016/j.neubiorev.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Chaby LE, Cavigelli SA, White A, Wang K, Braithwaite VA. Long-term changes in cognitive bias and coping response as a result of chronic unpredictable stress during adolescence. Front Hum Neurosci. 2013;7:328. doi: 10.3389/fnhum.2013.00328. 10.3389/fnhum.2013.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Destrez A, Coulon M, Deiss V, Delval E, Boissy A, Boivin X. The valence of the long-lasting emotional experiences with various handlers modulates discrimination and generalization of individual humans in sheep. J Anim Sci. 2013;91:5418–5426. doi: 10.2527/jas.2012-5654. 10.2527/jas.2012-5654. [DOI] [PubMed] [Google Scholar]
- 66.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1083–1090. doi: 10.1016/j.biopsych.2009.06.006. 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, et al. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer FK, Greve DN, et al. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience. 2014;266:1–12. doi: 10.1016/j.neuroscience.2014.01.058. 10.1016/j.neuroscience.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis AH, Porcelli AJ, Delgado MR. The effects of acute stress exposure on striatal activity during Pavlovian conditioning with monetary gains and losses. Front Behav Neurosci. 2014;8:179. doi: 10.3389/fnbeh.2014.00179. 10.3389/fnbeh.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol Psychiatry. 2012;72:157–163. doi: 10.1016/j.biopsych.2012.03.014. 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Porcelli AJ, Lewis AH, Delgado MR. Acute stress influences neural circuits of reward processing. Front Neurosci. 2012;6:157. doi: 10.3389/fnins.2012.00157. 10.3389/fnins.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi JM, Padmala S, Spechler P, Pessoa L. Pervasive competition between threat and reward in the brain. Soc Cogn Affect Neurosci. 2014;9:737–750. doi: 10.1093/scan/nst053. 10.1093/scan/nst053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grupe DW, Oathes DJ, Nitschke JB. Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb Cortex. 2013;23:1874–1883. doi: 10.1093/cercor/bhs175. 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, Pessoa L. Network organization unfolds over time during periods of anxious anticipation. J Neurosci. 2014;34:11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 80.Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- 81.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- 83.Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am J Psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 85.Ganzel B, Casey BJ, Glover G, Voss HU, Temple E. The aftermath of 9/11: effect of intensity and recency of trauma on outcome. Emotion. 2007;7:227–238. doi: 10.1037/1528-3542.7.2.227. 10.1037/1528-3542.7.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 87.Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, et al. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54:233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 88.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 89.van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 92.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 93.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 94.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang SE, Wheeler DS, Holland PC. Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. Neurobiol Learn Mem. 2012;97:441–451. doi: 10.1016/j.nlm.2012.03.008. 10.1016/j.nlm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simmons DA, Neill DB. Functional interaction between the basolateral amygdala and the nucleus accumbens underlies incentive motivation for food reward on a fixed ratio schedule. Neuroscience. 2009;159:1264–1273. doi: 10.1016/j.neuroscience.2009.01.026. 10.1016/j.neuroscience.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 97.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dong H, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- 99.Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 100.Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J Comp Neurol. 2003;463:434–472. doi: 10.1002/cne.10758. 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- 101.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471:396–433. doi: 10.1002/cne.20002. 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- 102.Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, et al. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci. 2012;32:18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Erb S, Shaham Y, Stewart J. Stress-induced relapse to drug seeking in the rat: role of the bed nucleus of the stria terminalis and amygdala. Stress. 2001;4:289–303. doi: 10.3109/10253890109014753. [DOI] [PubMed] [Google Scholar]
- 106.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 108.Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wenzel JM, Waldroup SA, Haber ZM, Su ZI, Ben-Shahar O, Ettenberg A. Effects of lidocaine-induced inactivation of the bed nucleus of the stria terminalis, the central or the basolateral nucleus of the amygdala on the opponent-process actions of self-administered cocaine in rats. Psychopharmacology (Berl) 2011;217:221–230. doi: 10.1007/s00213-011-2267-7. 10.1007/s00213-011-2267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]