Abstract
Background
Grip testing is commonly used as an objective measure of strength in the hand and upper extremity and is frequently used clinically as a proxy measure of function. Increasing knowledge of hand biomechanics, muscle strength, and prehension patterns can provide us with a better understanding of the functional capabilities of the hand. The objectives of this study were to determine the contribution of ulnar digits to overall grip strength in individuals with thumb carpometacarpal (CMC) osteoarthritis (OA).
Methods
Thirty-seven subjects participated in the study. This group consisted of 19 patients with CMC OA (aged 60–88 years) and 18 healthy subjects (60–88 years). Three hand configurations were used by the subjects during grip testing: use of the entire hand (index, middle, ring, and little fingers) (IMRL); use of the index, middle, and ring fingers (IMR); and use of only the index and middle fingers (IM).
Results
Grip strength findings for the two groups found that compared to their healthy counterparts, CMC OA patients had, on average, a strength deficiency of 45.6, 35.5, and 28.8 % in IMRL, IMR, and IM, respectively. The small finger contribution to grip is 14.3 % and the ring and small finger contribute 34 % in subjects with CMC OA.
Discussion
Grip strength decreases as the number of digits contributing decreased in both groups. The ulnar digits contribution to grip strength is greater than one third of total grip strength in subjects with CMC OA. Individuals with CMC OA demonstrate significantly decreased grip strength when compared to their healthy counterparts.
Keywords: Thumb, Carpometacarpal osteoarthritis, Grip strength, Hand therapy, Occupational therapy, Physical therapy
Introduction
Grip strength is an objective measure of function in the hand and upper extremity. Physicians and hand therapists can establish a baseline, assess progress, and evaluate outcomes after surgery or other therapeutic interventions and can use it as an outcome measure. Gehrmann et al. studied the effects of severe carpometacarpal (CMC) osteoarthritis (OA) on the three-dimensional motion capability of the thumb and found severe stages of thumb CMC OA that causes an asymmetrical motion deficit with decreased range of motion (ROM) in extension and adduction, leading to decreased capability of counteropposition [5]. They also report the functional consequence of an arthritic thumb’s motion envelope that the thumb cannot easily release its grip of an object [5]. A thumb with advanced CMC OA that is adducted will also have difficulty gripping an object due to the adduction deformity. Nunes et al. demonstrated a strong positive correlation between hand function test outcomes and grip force control in individuals with hand OA [14]. They also established that individuals with hand OA have increased latency (the time between grabbing an object and moving it) and this may interfere with an individual’s ability to perform everyday manual tasks such as turning a key, writing, or using a knife to prepare food [14]. The study also found no correlation between pain and the parameters of grip force [14].
Power grip occurs as the digits flex, rotate, and ulnarly deviate to compress an object held in the hand using the thumb as a buttress [10]. Past research suggests that the radial digits may be stronger than the ulnar-sided digits [21]. Although MacDermid et al. [10] concluded that the ulnar side of the hand contributes a smaller proportion of overall grip (approximately 60 % radial, 40 % ulnar), activation of muscle units in the little finger can produce relatively large forces, suggesting that the little finger contributes significantly to force production as well [9]. Bagis et al. [2] reported a statistically significant lower grip strength in subjects with grade IV OA. The contribution of the fourth and fifth fingers to grip strength in thumb carpometacarpal (CMC) OA has not been verified to date. This could be clinically relevant, since a larger contribution of the last unaffected fingers could compensate for the deficiency of the thumb due to pathological process. Conversely, in case of reduction of strength of the ring and last fingers, presumably due to inefficient usage, this could be targeted with specific rehabilitation strategies.
The purpose of this paper is to examine grip strength in patients with CMC OA when the ulnar digits are restricted. It is hypothesized that the ulnar digits make a significant contribution to functional grip strength. This study aims to determine the contribution of the little finger, and the little and ring finger together in overall grip strength in patients with CMC OA. The measurements were also performed in healthy subjects of a similar age range so a comparison can be drawn between healthy subjects and those with CMC OA.
Materials and Methods
Participants
A sample size calculation was performed to determine the necessary number of subjects needed for this study based upon the results of a previous pilot study [24–26]. A convenience sample of 37 right hand-dominant subjects, aged from 60 to 90 years old, were screened for eligibility criteria at the Department of Physical Therapy, Residenza Sanitaria Assistenziale “A. Maritano,” Sangano, Italy. Informed consent was obtained from all participants and procedures were conducted according to the Declaration of Helsinki. This group consisted of 19 subjects (age range 60–88 years; mean ± SD of 79 ± 8.2) diagnosed with CMC OA and 18 healthy subjects (age range 60–88 years; mean ± SD of 74 ± 7.8). CMC OA was confirmed by a hand surgeon examination of the participant’s hand X-ray. Inclusion criteria consisted of subjects who used their dominant hand on a regular basis (e.g., ex-factory workers and home workers) and diagnosed with CMC OA in the dominant hand by X-ray detection of stage III–IV according to the Eaton-Littler-Burton Classification [7, 26]. Subjects were excluded if they scored >4 points in the Beck Depression Inventory (BDI) [3] or more than 30 points in the State Trait Anxiety Inventory (STAI) [1]. Subjects with hand OA in the proximal or distal interphalangeal joints of the fingers of the tested hand were also excluded, as the presence of finger OA, particularly Heberden’s nodes, has been shown to decrease grip strength [2, 8, 12, 17].
Subjects with a medical history of carpal tunnel syndrome, surgical interventions to the thumb CMC joint, or De Quervain’s tenosynovitis, as well as those presenting with any neurological condition in which pain perception was altered were also excluded. The healthy subjects were free of any hand pathology, including hand OA, as verified by a hand surgeon following physical examination and review of hand X-rays.
Study Design and Procedures
The study protocol was identical for subjects and healthy controls. All examinations were performed in a quiet and draft-free laboratory. Participants were asked not to take analgesics, muscle relaxants, or anti-inflammatory drugs for 48 h before the examination. Participants sat in a comfortable sitting position with the dominant arm resting over a table. They were allowed to familiarize themselves with the grip dynamometer tool prior to testing.
Grip strength measurements were taken with a grip dynamometer (Baseline, NY, USA), which has a precision and reliability of ±3 % [19–21]. The grip dynamometer has five settings representing grip spans; however, only position two was used during this study protocol, since this has been shown to be the most reliable to obtain maximal grip strength for clinical and research purposes [3, 4]. Subjects were given standardized instructions for each grip test measurement (seated position with the shoulder adducted and neutrally rotated, the elbow flexed to 90°, and the forearm and wrist in a neutral position) [18, 19, 23].
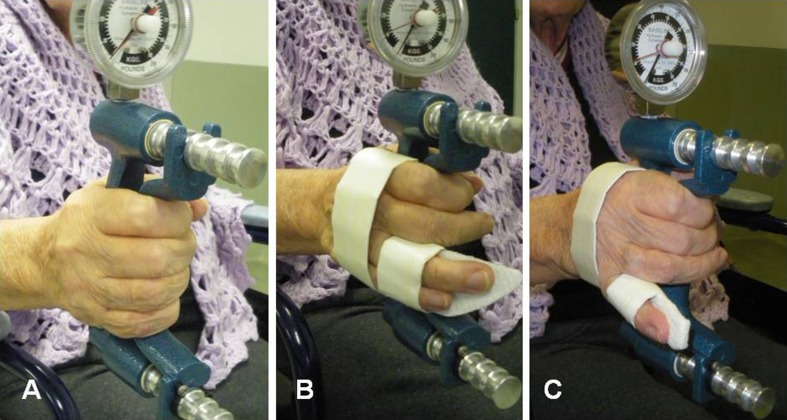
Subjects were tested using three different hand configurations: index, middle, ring, and little fingers (IMRL); index, middle, and ring fingers (IMR); and index and middle fingers (IM). Fingers excluded from test were held in place by orthotic devices that held the metacarpal phalangeal joint and proximal interphalangeal joint in full extension and the distal interphalangeal joint in a neutral position (see Fig. 1) [12]. There was no orthotic material in the palm to hinder gripping of the thumb, index, or long fingers. The order of testing was consistent, with three measurements taken, with a 1-min pause between measurements for each hand configuration. The mean of these three trials was used for analysis. The testing procedure took approximately 20 min to complete.
Fig. 1.
Finger orthotics immobilizing digits not performing the grip test: a IMRL: index, middle, ring, and little; b IMR: index, middle, and ring; c IM: index and middle
Data Analysis
Data were analyzed with SPSS statistical package (version 20, IBM, NY, USA). The data were analyzed using a 3 × 2 repeated measures analysis of variance (ANOVA) between the groups (patients and controls) and within the group between the different grip configurations (IMRL, IMR, and IM fingers) as the within-subject factors. Significant interaction terms were further parsed by examining simple main effects. All univariate analyses were evaluated using the Greenhouse-Geisser epsilon correction for the lack of sphericity. Post hoc evaluations of significant main effects were conducted using Bonferroni-adjusted pairwise comparisons. Furthermore, the differences between configurations (IMRL, IMR, and IM fingers) in the groups and mean grip strength using all digits between groups were estimated using the Cohen effect size (d). An effect size greater than 0.8 was considered large, around 0.5 moderate, and less than 0.2 small. Effect size measures were presented as partial eta-squared, which can be interpreted as the percentage of variance accounted for by an effect after controlling for other factors in the model. The statistical analysis was conducted at a 95 % confidence level, and a P < 0.05 was considered statistically significant.
Results
Table 1 presents the baseline demographics for both groups. The grip strength findings for the two groups indicate that compared to their healthy counterparts, CMC OA patients had, on average, a strength deficiency of 45.6, 35.5, and 28.8 % in IMRL, IMR, and IM, respectively. Mean grip strength using all digits of normal subjects was 20.6 kg (SD 7.3) compared to the mean grip of 11.2 kg (SD 6.3) of individuals with CMC OA. Effect size of between-group means was large 1.38.
Table 1.
Baseline demographics for both groups
| Configurations | Number | Median | Minimum | Maximum | Mean ± SD |
|---|---|---|---|---|---|
| Healthy group | |||||
| Age | 18 | 72.5 | 60 | 88 | 74.2 ± 7.8 |
| IMRL | 18 | 17.5 | 14 | 35 | 20.6 ± 7.3 |
| IMR | 18 | 14.7 | 8 | 28 | 15.0 ± 5.7 |
| IM | 18 | 10.0 | 6.0 | 18.0 | 10.4 ± 3.5 |
| CMC OA group | |||||
| Age | 19 | 81 | 60 | 88 | 78.8 ± 8.2 |
| IMRL | 19 | 10.0 | 3.0 | 29.0 | 11.2 ± 6.3 |
| IMR | 19 | 8.0 | 4.0 | 24.0 | 9.6 ± 5.9 |
| IM | 19 | 7.0 | 4.0 | 17.0 | 7.4 ± 3.8 |
Data are expressed as means ± standard deviation (SD)
IMRL index, middle, ring, and little; IMR index, middle, and ring; IM index and middle
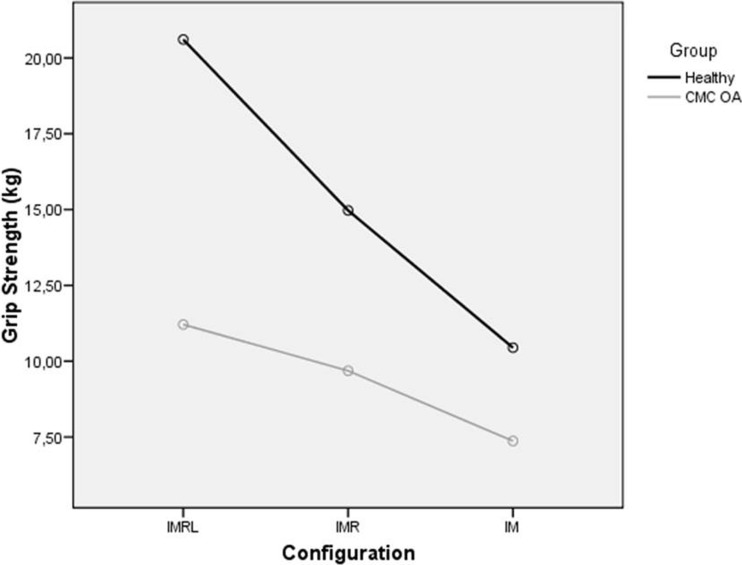
Univariate results demonstrated that grip strength was significantly predicted by the interaction between group and configuration (F[1.392] = 9.322; P = 0.001; partial eta = 0.2). The mean plot for the interaction term is displayed in Fig. 2. Similarly, significant main effects were demonstrated for grip configuration (F[1.392] = 44.405; P < 0.001; partial eta = 0.6).
Fig. 2.
Effect of configuration on grip strength. IMRL index, middle, ring, and little; CMC carpometacarpal
Post hoc evaluation of the simple main effect of configuration yielded a statistically significant difference between each level of grip configuration in the healthy group, with grip strength decreasing as the number of digits contributing decreased (all, P < 0.01). Similarly, significant main effects demonstrated between IMRL and IM (P = 0.01) and IMR and IM (P = 0.002) were detected in the patient group. The abovementioned post hoc comparisons are presented in Tables 2 and 3. Between-group effect sizes were large (between d = 1.38 and d = 0.84) in IMRL, IMR, and IM.
Table 2.
Bonferroni-adjusted pairwise comparisons
| Comparison | Mean difference | P | 95 % CI for difference | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Healthy group | ||||
| IMRL - IMR | 5.6a | <0.001 | 2.6 | 8.7 |
| IMRL - IM | 10.2a | <0.001 | 7.0 | 13.3 |
| IMR - IM | 4.5a | <0.001 | 3.0 | 6.1 |
| CMC OA group | ||||
| IMRL - IMR | 1.5 | 0.6 | −1.4 | 4.5 |
| IMRL - IM | 3.8a | 0.01 | 0.8 | 6.9 |
| IMR - IM | 2.3a | 0.002 | 0.8 | 3.8 |
Data are expressed as means ± standard deviation (SD)
CMC carpometacarpal; IMRL index, middle, ring, and little; IMR index, middle, and ring; IM index and middle
aSignificant differences between both groups
Table 3.
IMRL, IMR, IM, and between-group differences for grip strength
| Grip strength | |||
|---|---|---|---|
| Group | IMRL | IMR | IM |
| Healthy group | 20.6 ± 1.6 | 15.0 ± 1.4 | 10.4 ± 0.9 |
| CMC OA group | 11.2 ± 1.6 | 9.7 ± 1.3 | 7.4 ± 0.8 |
| Between group differences | 9.4 (4.9/13.9) | 5.3 (1.4/9.2) | 3.1 (0.6/5.5) |
| P | <0.001a | 0.009a | 0.015a |
Data are expressed as means ± standard deviation (SD)
IMRL index, middle, ring, and little; IMR index, middle, and ring; IM index and middle; CMC carpometacarpal
aSignificant differences between both groups
The contribution of grip strength by the small finger for individuals with CMC OA was calculated by dividing the mean grip when the small finger was excluded (9.6 kg) by the mean grip measured when all digits were tested (11.2 kg) and then calculating the difference by subtracting 85.7 from 100 %. The small finger represents 14.3 % of the grip in individuals with CMC OA. The contribution of both the small and ring fingers was calculated in the same manner. The contribution of the ring and small fingers represents 34 % of the grip in individuals with CMC OA.
The contribution of grip strength by the small finger for normal subjects was calculated by dividing the mean grip measured when the small finger was excluded (15.0 kg) by the mean grip when all digits were tested (20.6) and then calculating the difference by subtracting 72.8 % from 100 %. The small finger represents 27.2 % of the grip in normal subjects. The contribution of both the small and ring fingers was calculated in the same manner. The contribution of the ring and small fingers represents 49.5 % of the grip in normal subjects.
Discussion
In the present study, grip strength scores were compared between healthy subjects and CMC OA patients. In addition, the contribution of the ulnar digits to overall grip strength was compared in both groups. The percentage of difference between grip strength in normal subjects and those with CMC OA (45.6 %) is in agreement with Bagis’ [2] study that reported individuals with hand OA have decreased strength compared to their healthy counterparts. Our study further defines the effect size of the differences between healthy subjects and individuals with CMC OA as being large (1.34), clarifying the differences between normal subject’s grip strength and those with CMC OA, demonstrating that those with CMC OA will have increased functional limitations due to diminished grip strength. The thumb adduction deformities associated with advanced CMC OA can result in a mechanical disadvantage and make the tasks of grasping and handling objects more difficult.
Ring and little finger contribution during normal grip strength has been reported to be between 22 and 28 % and 15 and 24 %, respectively, in normal subjects [15, 17, 22]. Ring and little finger contribution of grip strength in subjects with CMC OA was calculated by comparing the decrease in strength when little and ring fingers were excluded and when just the little finger was excluded. The reduction in mean grip strength measured with all digits in the subjects with CMC OA decreased by 34 % when the little and ring fingers were removed and 14.3 % when just the little finger was removed. The little and ring fingers contributed 49.5 % to the grip strength of normal subjects, whereas the little finger contributed 27 % to grip strength in normal subjects. In this study, ulnar-sided digits provided greater than one third of the grip of individuals with CMC OA but nearly one half of the grip strength of normal subjects. Therefore, we demonstrated that the ulnar-sided digit contribution to grip is meaningful but variable between healthy subjects and individuals with CMC OA. It has previously been reported that every finger exerts maximal strength when working alone, but exerts decreased strength in proportion to the number of other fingers working together [16]. It would be difficult to quantify the exact contribution of each digit, because as fingers are removed from the grip testing, some over compensation of the other digits probably occurs. Interestingly, it has been reported that the individual finger contribution to the total grip force changes with weight and diameter of the object being grasped and that the thumb contribution always exceeded 38 %, followed by the ring and small fingers, which contributed between 18 and 23 % for all weights and diameters [18]. Anticipation of the grip task that will be accomplished has also been shown to alter the contribution of the individual digits [11, 22]. Because individuals with CMC OA have decreased contribution of the ulnar digits, this causes increased load to be borne by the thumb. It has been reported that applied forces to the CMC joint may reach 20–25 kg times the applied load [11]. This can further contribute to CMC deformity and pathology. When load forces are taken into consideration with the findings of this study that found diminished contribution of the ulnar side of the hand to bear load, the clinician should apply repetitive gripping exercises very judiciously. In addition, this population has been found to have proprioceptive deficits [6, 13]. Hence, rehabilitation programs that include proprioception training for object manipulation may be useful for improving hand function and dexterity in individuals with CMC OA.
Limitations
This study included individuals with dominant hand grades 3 and 4 CMC OA, and therefore, the findings of the study cannot be generalized to a population of patients with grades 1 and 2 CMC OA or to subjects’ non-dominant hands. The relatively small sample size can also diminish the ability to generalize the results of this study. Another limitation of the study was that isometric grip strength was tested using a standardized position and a dynamometer, and the results may have been different if grip strength testing was performed during various functional tasks using concentric muscle contractions and was being evaluated by a pressure-sensitive electrodes rather than a dynamometer. Muscle recruitment and the force required to complete a task may be very different in the normal everyday accomplishment of activities of daily living compared to the forces required during standardized testing performed in a laboratory.
Conclusions
This is the first study of its kind that looked at the contribution of ulnar-sided digits to overall grip strength in subjects with CMC OA. It was determined that grip strength decreases as the number of digits contributing decreased in both the subjects with CMC OA and the normal group. The ulnar digits significantly contribute to grip strength in subjects with and without CMC OA. Despite their contribution to overall grip strength, subjects with CMC OA have significantly decreased grip strength as compared to their healthy counterparts and will likely suffer functional consequences due to this limitation. Future studies should explore the effect of randomizing the testing protocol and the effect of restriction of the ulnar digits during dynamic grip testing. Because the contribution of the ulnar digits with individuals with CMC OA is less than normal subjects, the applied load to the CMC joint of gripping activities should be considered when clinicians prescribe repetitive gripping tasks to build strength as the forces that occur at the CMC joint can cause further damage to the joint.
Acknowledgments
Conflict of Interest
Jorge H. Villafañe declares that he has no conflict of interest.
Kristin Valdes declares that he has no conflict of interest.
Santiago Angulo-Diaz-Par declares that he has no conflict of interest.
Paolo Pillastrini declares that he has no conflict of interest.
Stefano Negrini declares that he has no conflict of interest.
Statement of Human and Animal Rights
Ethical approval of the study was received by the institutional local board review. All procedures were conducted according to the Declaration of Helsinki of 1975, as revised in 2000 (5).
Statement of Informed Consent
Informed consent was obtained from all participants and all procedures were conducted according to the Declaration of Helsinki.
Contributor Information
Jorge H. Villafañe, Phone: +39.011. 9065495, Email: mail@villafane.it
Kristin Valdes, Email: hotglassgal@comcast.net.
Santiago Angulo-Diaz-Parreño, Email: sangulo@ceu.es.
Paolo Pillastrini, Email: paolo.pillastrini@unibo.it.
Stefano Negrini, Email: stefano.negrini@med.unibs.it.
References
- 1.Antunes HK, Stella SG, Santos RF, Bueno OF, de Mello MT. Depression, anxiety and quality of life scores in seniors after an endurance exercise program. Rev Bras Psiquiatr. 2005;27:266–71. doi: 10.1590/S1516-44462005000400003. [DOI] [PubMed] [Google Scholar]
- 2.Bagis S, Sahin G, Yapici Y, Cimen OB, Erdogan C. The effect of hand osteoarthritis on grip and pinch strength and hand function in postmenopausal women. Clin Rheumatol. 2003;22:420–4. doi: 10.1007/s10067-003-0792-4. [DOI] [PubMed] [Google Scholar]
- 3.Beck AT, Steer RA, Ranieri WF. Scale for suicide ideation: psychometric properties of a self-report version. J Clin Psychol. 1988;44:499–505. doi: 10.1002/1097-4679(198807)44:4<499::AID-JCLP2270440404>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Firrell JC, Crain GM. Which setting of the dynamometer provides maximal grip strength? J Hand Surg [Am] 1996;21:397–401. doi: 10.1016/S0363-5023(96)80351-0. [DOI] [PubMed] [Google Scholar]
- 5.Gehrmann SV, Tang J, Zong ML, Goitz RJ, Windolf J, Kaufmann RA. Motion deficit of the thumb in CMC joint arthritis. J Hand Surg. 2010;35:1449–53. doi: 10.1016/j.jhsa.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Hagert E, Lee J, Ladd AL. Innervation patterns of thumb trapeziometacarpal joint ligaments. J Hand Surg. 2012;37:706–14. doi: 10.1016/j.jhsa.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Jaggi R, Morris S. Practice tips. Rule of thumb: update on first carpometacarpal joint osteoarthritis. Can Fam Physician. 2007;53:1309–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Jones G, Cooley HM, Bellamy N. A cross-sectional study of the association between Heberden’s nodes, radiographic osteoarthritis of the hands, grip strength, disability and pain. Osteoarthr Cartil. 2001;9:606–11. doi: 10.1053/joca.2001.0460. [DOI] [PubMed] [Google Scholar]
- 9.Kilbreath SL, Gorman RB, Raymond J, Gandevia SC. Distribution of the forces produced by motor unit activity in the human flexor digitorum profundus. J Physiol. 2002;543:289–96. doi: 10.1113/jphysiol.2002.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDermid JC, Lee A, Richards RS, Roth JH. Individual finger strength: are the ulnar digits “powerful”? J Hand Ther. 2004;17:364–7. doi: 10.1197/j.jht.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Marshall M, van der Windt D, Nicholls E, Myers H, Hay E, Dziedzic K. Radiographic hand osteoarthritis: patterns and associations with hand pain and function in a community-dwelling sample. Osteoarthr Cartil. 2009;17:1440–7. doi: 10.1016/j.joca.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Methot J, Chinchalkar SJ, Richards RS. Contribution of the ulnar digits to grip strength. Can J Plast Surg. 2010;18:e10–4. [PMC free article] [PubMed] [Google Scholar]
- 13.Morbargha N, Ludwig C, Ladd AL, Hagert E. Ultrastructure and innervation of thumb carpometacarpal ligaments in surgical patients with osteoarthritis. Clin Orthop Relat Res. 2013 doi: 10.1007/s11999-013-3083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes PM, de Oliveira DG, Aruin AS, dos Santos MJ. Relationship between hand function and grip force control in women with hand osteoarthritis. J Rehabil Res Dev. 2012;49:855–65. [PubMed] [Google Scholar]
- 15.Ohtsuki T. Inhibition of individual fingers during grip strength exertion. Ergonomics. 1981;24:21–36. doi: 10.1080/00140138108924827. [DOI] [PubMed] [Google Scholar]
- 16.Ozkan B, Keskin D, Bodur H, Barca N. The effect of radiological hand osteoarthritis on hand function. Clin Rheumatol. 2007;26:1621–5. doi: 10.1007/s10067-007-0555-8. [DOI] [PubMed] [Google Scholar]
- 17.Radhakrishnan S, Nagaravindra M. Analysis of hand forces in health and disease during maximum isometric grasping of cylinders. Med Biol Eng Comput. 1993;31:372–6. doi: 10.1007/BF02446690. [DOI] [PubMed] [Google Scholar]
- 18.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing. 2006;35:409–15. doi: 10.1093/ageing/afl024. [DOI] [PubMed] [Google Scholar]
- 19.Schweizer R, Martin DD, Schonau E, Ranke MB. Muscle function improves during growth hormone therapy in short children born small for gestational age: results of a peripheral quantitative computed tomography study on body composition. J Clin Endocrinol Metab. 2008;93:2978–83. doi: 10.1210/jc.2007-2600. [DOI] [PubMed] [Google Scholar]
- 20.Solanki PV, Mulgaonkar KP, Rao SA. Effect of early mobilisation on grip strength, pinch strength and work of hand muscles in cases of closed diaphyseal fracture radius-ulna treated with dynamic compression plating. J Postgrad Med. 2000;46:84–7. [PubMed] [Google Scholar]
- 21.Talsania JS, Kozin SH. Normal digital contribution to grip strength assessed by a computerized digital dynamometer. J Hand Surg (Edinburgh, Scotland) 1998;23:162–6. doi: 10.1016/S0266-7681(98)80165-4. [DOI] [PubMed] [Google Scholar]
- 22.Villafañe JH, Cleland JA, Fernandez-de-Las-Pena C. Bilateral sensory effects of unilateral passive accessory mobilization in patients with thumb carpometacarpal osteoarthritis. J Manipulative Physiol Ther. 2013;36:232–7. doi: 10.1016/j.jmpt.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Villafañe JH, Cleland JA, Fernandez-de-Las-Penas C. The effectiveness of a manual therapy and exercise protocol in patients with thumb carpometacarpal osteoarthritis: a randomized controlled trial. J Orthop Sports Phys Ther. 2013;43:204–13. doi: 10.2519/jospt.2013.4524. [DOI] [PubMed] [Google Scholar]
- 24.Villafañe JH, Silva GB, Diaz-Parreno SA, Fernandez-Carnero J. Hypoalgesic and motor effects of kaltenborn mobilization on elderly patients with secondary thumb carpometacarpal osteoarthritis: a randomized controlled trial. J Manipulative Physiol Ther. 2011;34:547–56. doi: 10.1016/j.jmpt.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Villafañe JH, Silva GB, Fernandez-Carnero J. Short-term effects of neurodynamic mobilization in 15 patients with secondary thumb carpometacarpal osteoarthritis. J Manipulative Physiol Ther. 2011;34:449–56. doi: 10.1016/j.jmpt.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Villafañe JH, Silva GB, Fernandez-Carnero J. Effect of thumb joint mobilization on pressure pain threshold in elderly patients with thumb carpometacarpal osteoarthritis. J Manipulative Physiol Ther. 2012;35:110–20. doi: 10.1016/j.jmpt.2011.12.002. [DOI] [PubMed] [Google Scholar]