FIGURE 2.
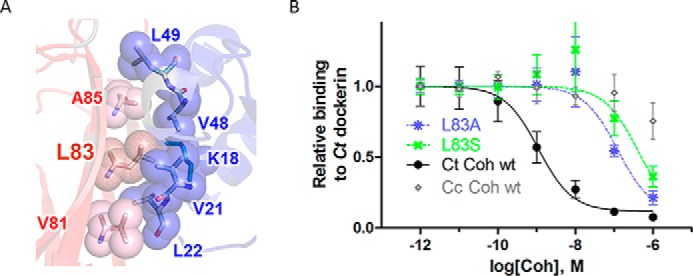
The hydrophobic patch contributes strongly to cohesin-dockerin binding affinity. A, detailed structural view of the hydrophobic interface patch. Residues involved in the cohesin (red)-dockerin (blue) interaction are shown in spheres and labeled (numbering according to Protein Data Bank code 1OHZ (12)) (see also Fig. 1C for sequence conservation of the hydrophobic patch). B, measurement of effect of C. thermocellum (Ct) cohesin mutations L83A (blue) and L83S (green) on binding to C. thermocellum dockerin using iELISA. Various concentrations of cohesin (x axis) were incubated with a constant concentration of dockerin in solution (0.1 nm), and the amount of unbound dockerin was detected on plates coated with wild-type cohesin (y axis; see “Experimental Procedures”). Binding wild-type C. thermocellum and C. cellulolyticum (Cc) cohesins to C. thermocellum dockerin represents positive (black) and negative (i.e. non-cognate, nonspecific; gray) binding controls, respectively. Results represent the average value over four independent experiments (the S.E. is shown as error bars).
