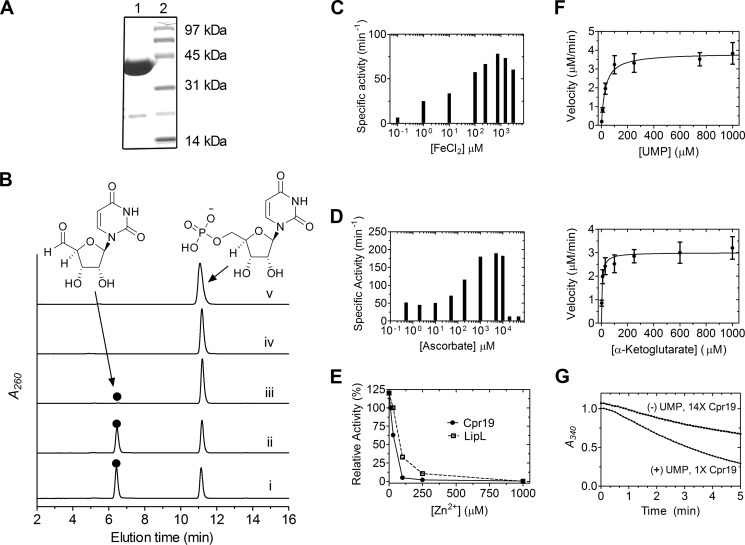
FIGURE 9.
In vitro characterization of the non-heme Fe(II)-dependent αKG:UMP dioxygenase Cpr19. A, SDS-PAGE of purified His6-Cpr19 (expected 35.3 kDa). The engineered N-terminal His6 tag contributes ∼5 kDa to the native molecular mass. B, HPLC analysis of the reaction catalyzed by Cpr19 using UMP as the prime substrate with all components (i) or the omission of ascorbic acid (ii), ferrous iron (iii), αKG (iv), or O2 (v). Similarly to LipL (27), Cpr19 co-purified with iron (4 ± 0.5%), explaining the formation of trace amounts of product without exogenous ferrous iron. ●, uridine-5′-aldehyde; A260, absorbance at 260 nm. C, optimal activity of Cpr19 with respect to varied Fe(II). D, optimal activity of Cpr19 with respect to varied ascorbic acid. E, activity with the addition of Zn2+ using the optimized reaction conditions. For comparison, the activity of LipL with Zn2+ is shown (from Ref. 27). F, plots for single-substrate kinetic analysis of CapH using optimized activity conditions. G, uncoupled oxidation of α-ketoglutarate to succinate in the absence of prime substrate (UMP), which for Cpr19 yielded a kcat = 1.6 min−1, corresponding to a relative turnover of 2.5% compared with the UMP-containing reaction. 14×, a 14-fold increase in enzyme utilized compared with data shown for 1× Cpr19. Error bars, S.D.