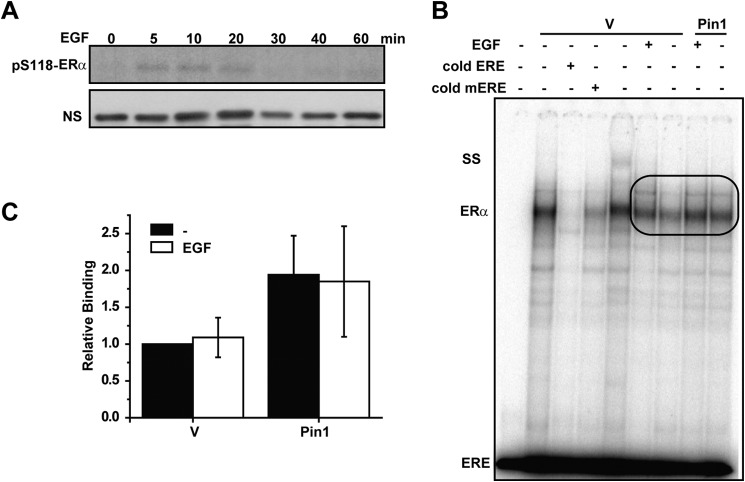
FIGURE 2.
Phosphorylation by EGF is insufficient to enhance Pin1-mediated effects on ERα-DNA binding. A, Western blot of ERα phosphorylated at Ser118 in response to 100 ng/ml of EGF at the indicated time points. NS, nonspecific band for loading control. B, nuclear extracts (10 μg) from MCF7 cells stably overexpressing GFP (vector (V)) or GFP-Pin1 (Pin1) treated with (EGF) and without (−) 100 ng/ml EGF were incubated with ERE followed by gel electrophoresis and autoradiography. The ERα-ERE complex was competed with unlabeled ERE or mERE. SS, ERα supershift using anti-ERα antibody. ERE represents unbound free probe. Bands in the oval box were quantified. C, EMSA autoradiography (B) was quantified using a PhosphorImager, and data are represented as means ± S.E. (error bars) for at least three independent experiments. No statistically significant differences were observed (p > 0.05).