FIGURE 4.
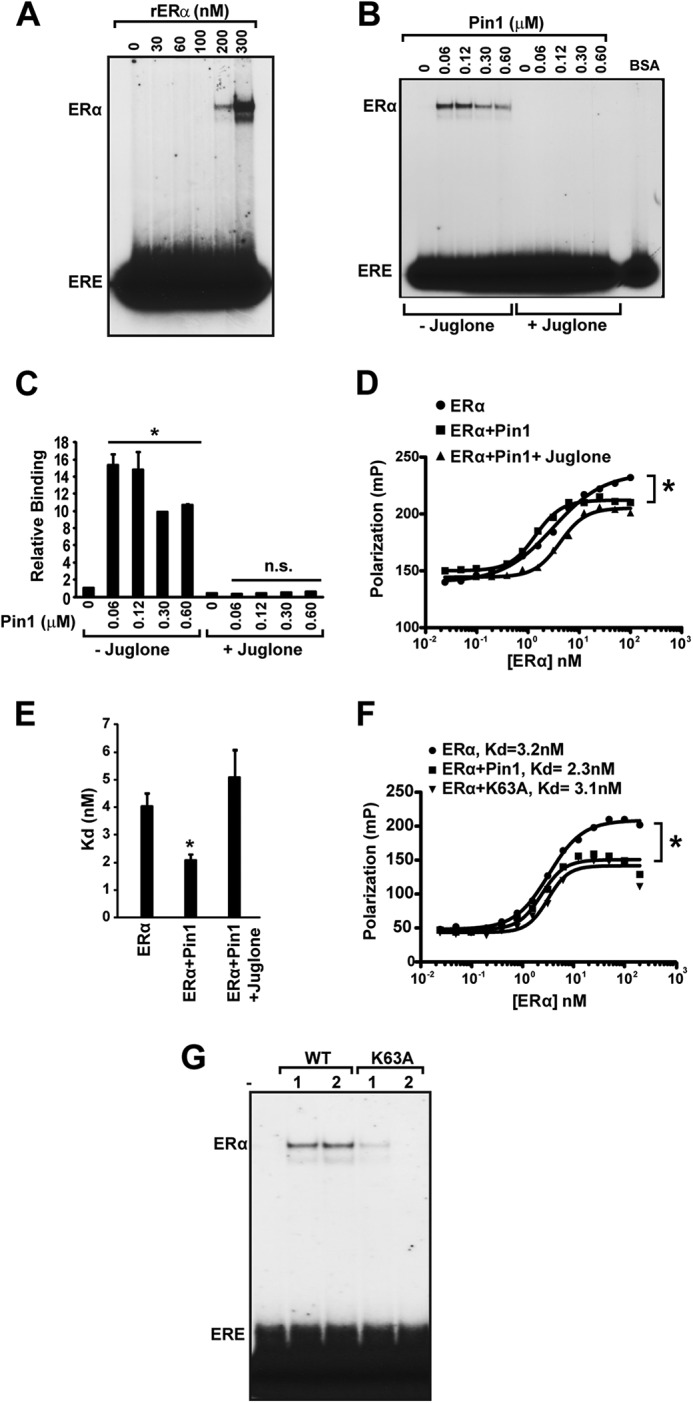
Pin1 isomerase function increases ERα binding affinity to ERE. A, to assess various levels of ERα-ERE binding, recombinant purified ERα (0–300 nm) was first incubated at 30 °C for 30 min and then incubated with 32P-labeled ERE, followed by gel electrophoresis and autoradiography. B, in vitro EMSA was performed as in (Fig. 3D) with 100 nm ERα and different Pin1 amounts (0–0.6 μm) in the presence and absence of Pin1 catalytic inhibitor, juglone (10 μm), and 100 ng of BSA. C, EMSA autoradiography in B was quantified using a PhosphorImager, and data are represented as means ± S.E. (error bars) for at least three independent experiments. *, p < 0.05; n.s., not significant comparing ERα and ERα + Pin1. D, determination of Kd values for unoccupied ERα (closed circles), ERα + Pin1 (closed squares), or ERα + Pin1 + juglone (closed triangles) for the ERE was assessed by FP. E, graph showing Kd values for ERα, ERα + Pin1, and ERα + Pin1 + juglone. Data are represented as means ± S.E. for at least three independent experiments. *, p < 0.05. F, determination of Kd values for unoccupied ERα (closed circles), ERα + Pin1 (closed squares), or ERα + K63A mutant Pin1 (closed triangles) for the ERE was assessed by FP, and a representative graph is shown. *, p < 0.05. G, in vitro EMSA was performed as in B in the presence and absence of 1 and 2 μm Pin1 (WT) or catalytic mutant K63A.
