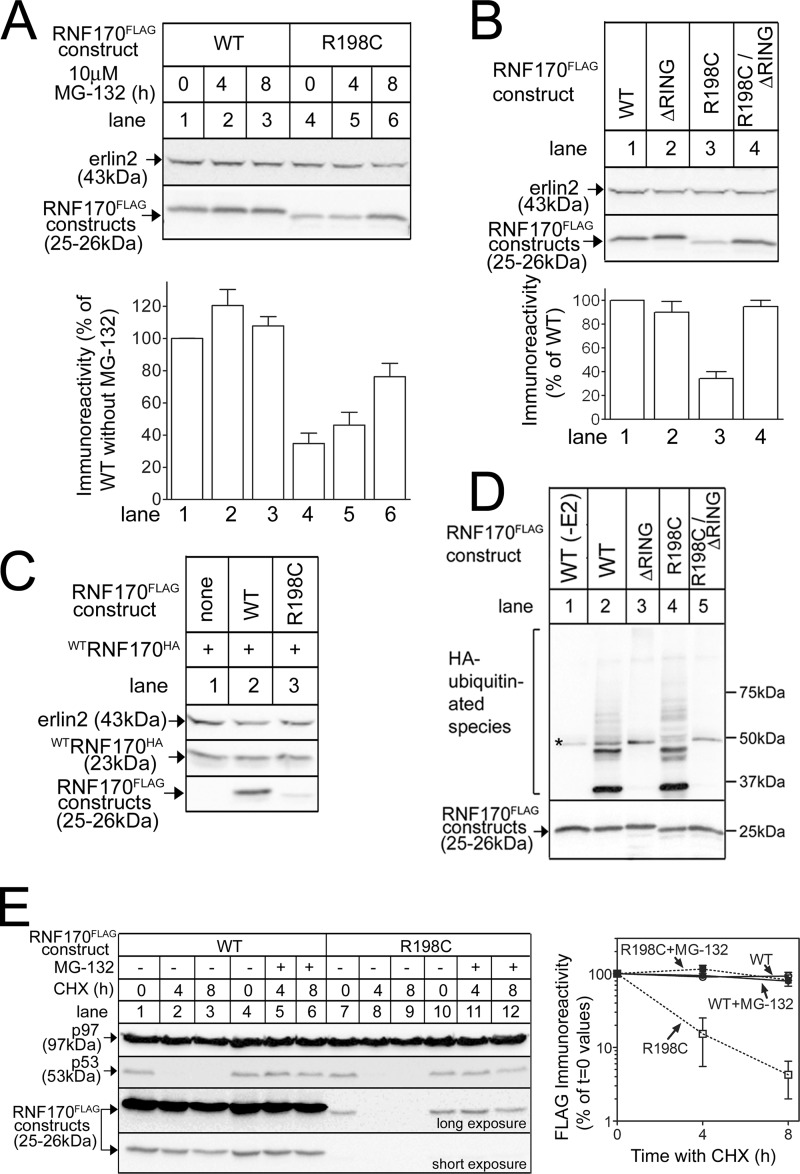
FIGURE 3.
The R198C mutation reduces RNF170 expression via autoubiquitination and the proteasome. A-C, cDNAs encoding tagged WT and mutant RNF170 constructs transfected into HeLa cells were treated as indicated, and cell lysates were probed with anti-FLAG or anti-HA to recognize exogenous RNF170 constructs, or anti-erlin2, which served a loading control. The histograms show combined quantitated immunoreactivity (mean ± S.E., n ≥ 4). D, RNF170FLAG constructs were immunopurified from transfected HeLa cells and incubated with E1 (UBE1), E2 (UbcH5b), and HA-ubiquitin as indicated for 30 min at 30 °C, with the exception of lane 1, which lacked E2. Samples were then probed with anti-HA to assess ubiquitination (upper panel), or anti-FLAG to assess the levels of RNF170FLAG constructs (lower panel). The asterisk marks a background band. E, transfected HeLa cells were treated as indicated with 20 μg/ml of cycloheximide (CHX), without or with 10 μm MG-132. Cell lysates were then probed with anti-p53 as a positive control for cycloheximide action, anti-p97 as a loading control, and anti-FLAG to recognize exogenous RNF170 constructs (long and short exposures are shown to facilitate visualization of immunoreactivity changes). The graph shows combined quantitated FLAG immunoreactivity, using the long exposure for R198CRNF170FLAG and the short exposure for WTRNF170FLAG (mean ± range, n = 2).