FIGURE 4.
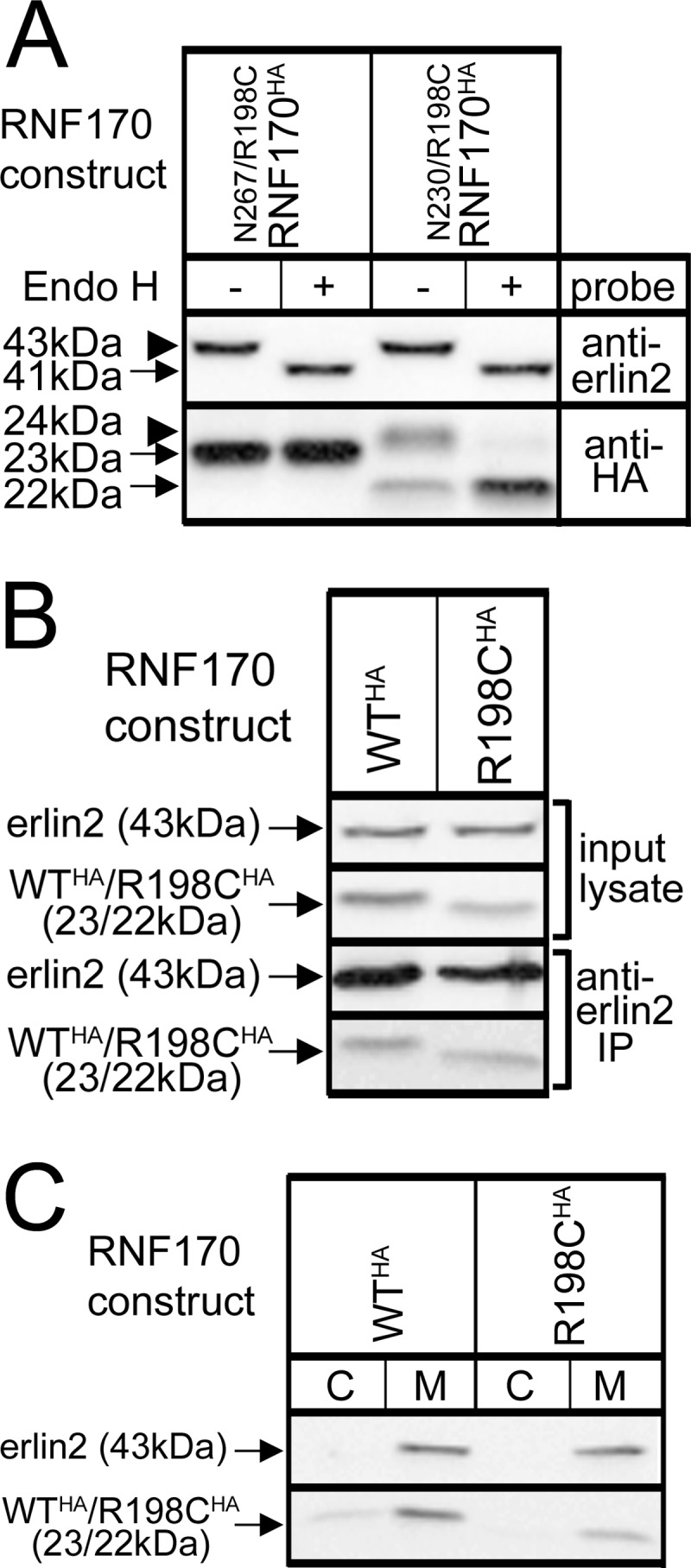
Lack of effect of the R198C mutation on RNF170 membrane association and topology, and interaction with the erlin1/2 complex. cDNAs encoding HA-tagged WT and mutant RNF170 constructs were transfected into HeLa cells. A, N-glycosylation of N267/R198CRNF170HA and N230/R198CRNF170HA, R198C-containing versions of the constructs shown in Fig. 1B, was assessed as described in the legend to Fig. 1B. B, interaction with the erlin1/2 complex. Erlin1/2 complex was immunoprecipitated with anti-erlin2 was probed for RNF170 constructs (lower panels). Note that the amounts of WTRNF170HA and R198CRNF170HA that co-immunoprecipitate are proportional to the amounts in input lysates, indicating that they interact with the erlin1/2 complex equally well. C, cells were lysed and centrifuged into cytosol (C) and membrane (M) factions as described (13), and probed as indicated.
