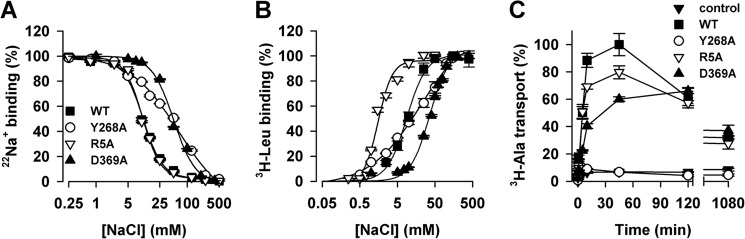
FIGURE 7.
Experimental measures of the interaction of Na+ with LeuT: effects of the perturbations. A, the binding of 1.92 μm [22Na]Cl by 0.8 pmol of LeuT-WT, LeuT-Y268A, LeuT-R5A, or LeuT-D369A was measured with 0–500 mm unlabeled NaCl in the absence of Leu. Data were normalized with respect to the maximum binding observed for each LeuT variant in the absence of non-labeled NaCl. Fitting of the isotopic 22Na+ replacement yielded an EC50 of 10.6 ± 0.5, 64.4 ± 17.2, 10.8 ± 0.6, and 49.9 ± 4.2 mm for LeuT-WT, -Y268A, -R5A, and -D369A, respectively, with Hill coefficients of 2.0 ± 0.2, 0.8 ± 0.1, 2.0 ± 0.2, and 1.8 ± 0.3. B, specific binding of [3H]Leu to LeuT-WT, -Y268A, -R5A, and -D369A was assayed in the presence of increasing NaCl concentrations. [3H]Leu concentrations were chosen to correspond to the Kd (see Fig. 8) and were 25 nm for LeuT-WT, 1 μm for LeuT-R5A and -Y268A, and 2 μm for LeuT-D369A. Fitting the data to the Hill equation revealed an EC50 of 8.9 ± 0.6, 12.3 ± 1.3, 1.5 ± 0.2, and 36.2 ± 2.1 mm with a Hill coefficient of 1.7 ± 0.2, 0.9 ± 0.1, 2.1 ± 0.1, and 1.7 ± 0.1 for LeuT-WT, -Y268A, -R5A, and -D369A, respectively. C, time course of 1 μm [3H]Ala uptake in proteoliposomes containing LeuT-WT (■), LeuT-Y268A (○), LeuT-R5A (▿), or LeuT-D369A (▴) and control liposomes lacking LeuT (▾) measured in the presence of 150 mm NaCl. Panels show representative experiments (n ≥ 2) performed in parallel; data points represent the mean ± S.E. of triplicate determinations. Kinetic constants were determined from the shown experiments with appropriate algorithms in GraphPad Prism version 5.01 or in Systat Software SigmaPlot version 10.0 and expressed as the mean ± S.E. of the fits.