FIGURE 1.
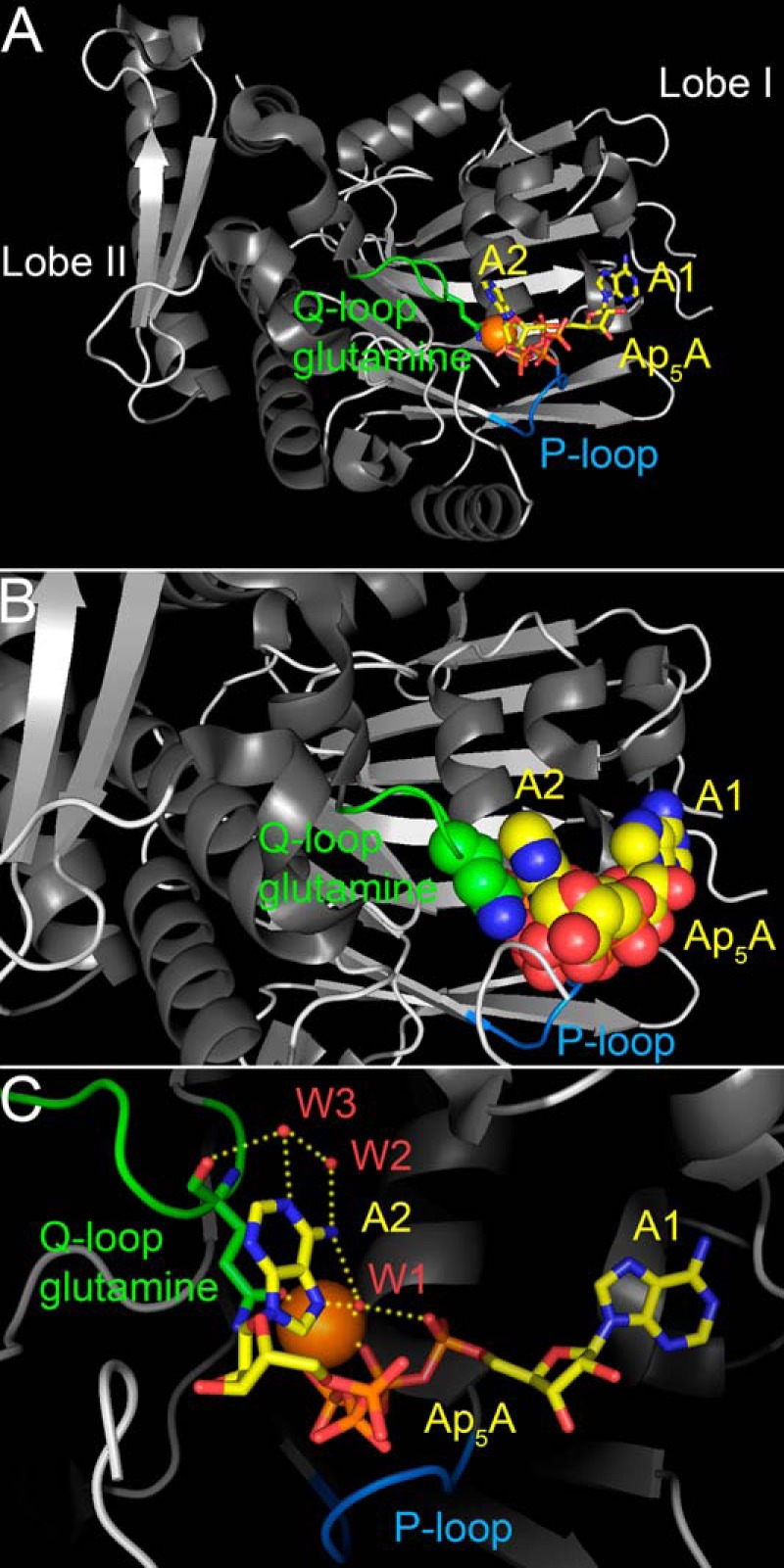
Structure of the NBD of the P. furiosus SMC protein in complex with Ap5A (Protein Data Bank code 3KTA) (19). A, one adenosine moiety of Ap5A (A1), the adjacent three phosphates, and a Mg2+ ion (orange sphere) are bound like Mg-ATP in complex with this SMC-NBD (71). The P-loop (Walker A motif) plays an essential role in phosphate group binding. The Q-loop and the position of the side chain of its conserved glutamine (Gln-145 of SMC) in relationship to the second adenosine moiety of Ap5A (A2) are depicted in green. The side-chain oxygen, which participates in Mg2+ coordination, is depicted in red, and the nitrogen is shown in blue. B, close-up view. The Q-loop glutamine side chain and the Ap5A molecule are shown in a space-filling representation to illustrate how the second adenosine moiety of Ap5A (A2) stacks onto the glutamine side chain. Water molecules contained in the crystal structure are not shown. C, several hydrogen bonds (yellow dotted lines) between Gln-145, the second adenosine moiety of Ap5A, and three water molecules (W1, W2, and W3; red spheres) are thought to confer specific recognition of adenine (19). Water-mediated base specificity has previously been observed in other nucleotide monophosphate kinases (85, 86).
