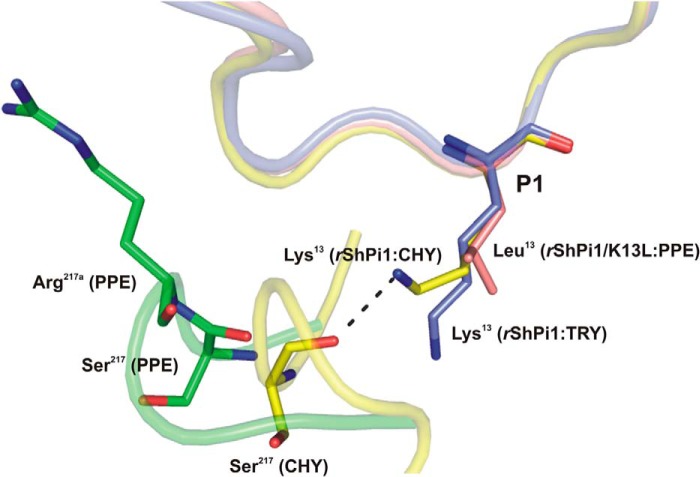
FIGURE 6.
Superposition of the P1 side chain conformation of rShPI-1/K13L (salmon) in complex with PPE (green) compared with that of the wild-type inhibitor in complexes with trypsin (blue, PDB code 3MTQ) and chymotrypsin (yellow, PDB code 3T62). The inhibitor binding loops are shown schematically with the P1 residues in sticks. The insertion of Arg217A in PPE triggers structural differences that prevent a stabilizing interaction of the basic P1 residue with Ser217, as observed in the chymotrypsin complex (32). Here, the side chain of Lys13 adopts an up conformation, which is different from the down conformation in the trypsin complex (blue) and is stabilized by an H-bond with the oxygen atom of Ser217. However, the insertion of Arg217A in PPE (green) moves Ser217 away from P1, suggesting that a similar stabilizing bond with basic residues at P1 is not possible.