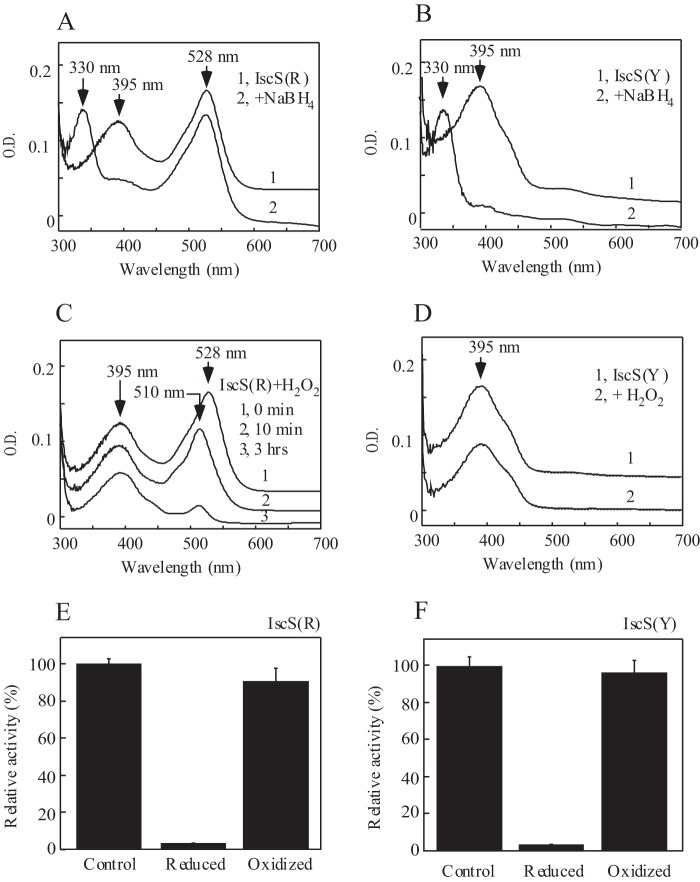
FIGURE 5.
Redox property of red IscS. A, reduction of red IscS by sodium borohydride. Purified red IscS (IscS(R), 30 μm, spectrum 1) was incubated with NaBH4 (100 μm, spectrum 2) at 4 °C for 30 min. B, reduction of wild-type IscS by sodium borohydride. Purified wild-type IscS (IscS(Y), 30 μm, spectrum 1) was incubated with NaBH4 (100 μm, spectrum 2) at 4 °C for 30 min. C, oxidation of red IscS by H2O2. Purified red IscS (30 μm, spectrum 1) was incubated with H2O2 (10 mm, spectrum 2) at 4 °C for 10 min (spectrum 2) or 3 h (spectrum 3). D, oxidation of wild-type IscS by H2O2. Purified wild-type IscS (30 μm, spectrum 1) was incubated with 10 mm H2O2 (spectrum 2) at 4 °C for 3 h. E, relative cysteine desulfurase activity of red IscS after being treated with sodium borohydride (reduced) or H2O2 (oxidized). F, relative cysteine desulfurase activity of wild-type IscS after being treated with sodium borohydride or H2O2. The enzyme activity of purified red IscS varied in different preparations and closely correlated with the amplitudes of the absorption peak at 395 nm of pyridoxal 5′-phosphate. The total enzyme activity of red IscS shown was ∼75% of that of wild-type IscS. The results are the representatives from three independent different experiments.