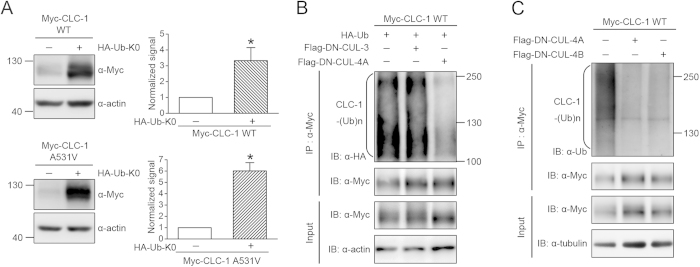
Figure 2. CUL4A and 4B regulate CLC-1 protein polyubiquitination.
(A) Biochemical demonstration of CLC-1 polyubiquitination in HEK293T cells. (Left) Representative immunoblots showing the effect of HA-tagged lysine-less ubiquitin (HA-Ub-K0) co-expression on Myc-CLC-1. (Right) Quantification of relative CLC-1 protein expression level. Standardized protein densities of the Ub-K0 co-expression group (hatched bars) were normalized to those for the corresponding HA-vector control (clear bars). Asterisks denote significant difference from the control (*, t-test: p < 0.05; n = 5-6).(B) CLC-1 polyubiquitination [CLC-1-(Ub)n] by HA-Ub was reduced by DN-CUL4A, but not DN-CUL3. Co-expression with the Flag vector was used as the control experiment. Cell lysates were immunoprecipitated (IP) with the anti-Myc antibody, and protein ubiquitination was recognized by immunoblotting (IB) the immunoprecipitates with the anti-HA antibody. Corresponding expression levels of CLC-1 and actin in the lysates are shown in the Input lane. In all cases hereafter, input represents about 10% of the total protein used for immunoprecipitation. (C) CLC-1 polyubiquitination by endogenous ubiquitin was disrupted in the presence of DN-CUL4A/B. Co-expression with the Flag vector was used as the control experiment. Protein ubiquitination was identified by immunoblotting the immunoprecipitates with the anti-ubiquitin (Ub) antibody. The gels were run under the same experimental conditions. Uncropped images of immunoblots are shown in Supplementary Figure S2.