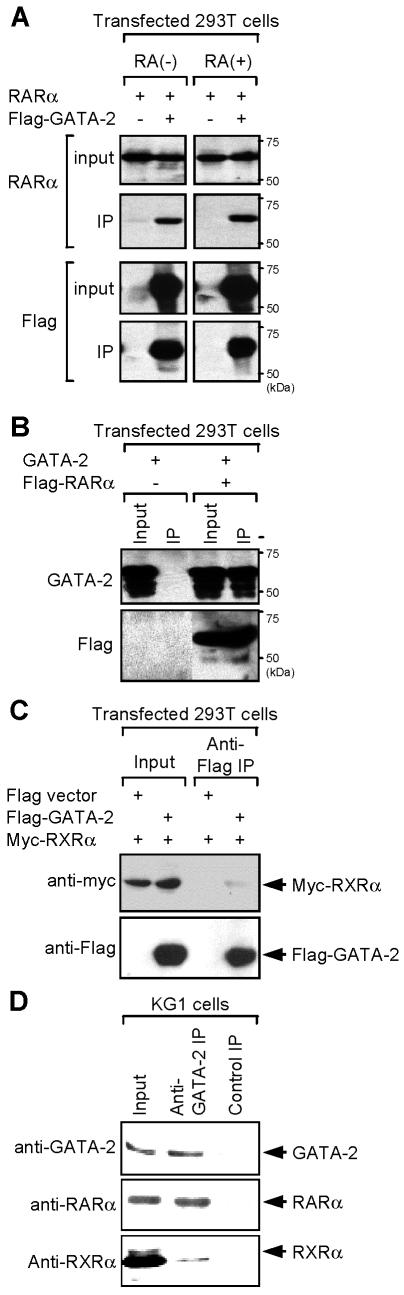
FIG. 2.
Coimmunoprecipitation analysis of GATA-2-RARα interaction. (A) 293T cells were transfected with expression plasmids encoding the indicated proteins and treated with 1 μM RA [RA(+)] or diluent alone [RA(−)] 24 h after transfection. Cell lysates were prepared 24 h later, immunoprecipitated (IP) with anti-Flag antibody, and analyzed by Western blotting with anti-RARα (top) or anti-Flag (bottom) antibodies. Input (10%) nuclear extracts were analyzed as controls for the level of protein expression. Note that under these conditions GATA-2 binds RARα, irrespective of RA treatment. (B) Lysates of 293T cells transfected with the indicated expression plasmids were immunoprecipitated with anti-Flag antibody and analyzed by anti-GATA-2 (top) or anti-Flag (bottom) antibodies. (C) Cell lysates of 293T cells transfected with the expression plasmids for the indicated proteins were immunoprecipitated with anti-Flag antibody as described above. The precipitated proteins were analyzed by Western blotting with the indicated antibodies. Note that RXRα only weakly binds GATA-2. (D) Nuclear extracts of human myeloid KG1 cells were immunoprecipitated with anti-GATA-2 antibody. The precipitated materials were then analyzed by Western blotting with antibodies against GATA-2, RARα, and RXRα. Mouse IgG was used as a control. Input (10%) materials were used as controls. Molecular size markers are indicated on the right.