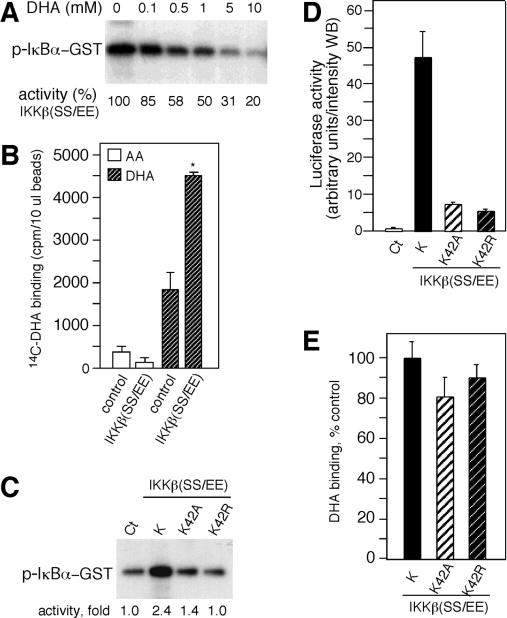
FIG. 5.
DHA binds to IKKβ. (A) The constitutively active IKKβ, IKKβ(SS/EE), immunoprecipitated from transfected cells was assayed in vitro for kinase activity. Kinase activity was determined by autoradiography. (B) IKKβ(SS/EE) bound to anti-FLAG beads was incubated with [14C]DHA and [14C]AA and eluted with FLAG peptide, and the radioactivity associated with IKKβ(SS/EE) was determined by scintillation spectrometry. The radioactivity nonspecifically associated with the anti-FLAG beads was estimated using beads incubated with extracts from nontransfected cells (control). (C) IKKβ(SS/EE) and the ATP binding site mutants IKKβ(SS/EE)K42A and K42R were assayed for kinase activity in vitro. A digitalized image was quantified, and the kinase activity is shown as fold increase below the blot. (D) HeLa cells cotransfected with pNFκB-luc reporter construct and IKKβ(SS/EE) (K), K42A, and K42R were assayed for luciferase activity. Luciferase activity is shown in arbitrary units per intensity of the Western blot (WB). Control extracts (Ct) were obtained from cells cotransfected with an empty vector. (E) Beads containing IKKβ(SS/EE) (K), K42A, and K42R were incubated with [14C]DHA, and its binding activity is shown as a percentage of control binding with IKKβ(SS/EE).