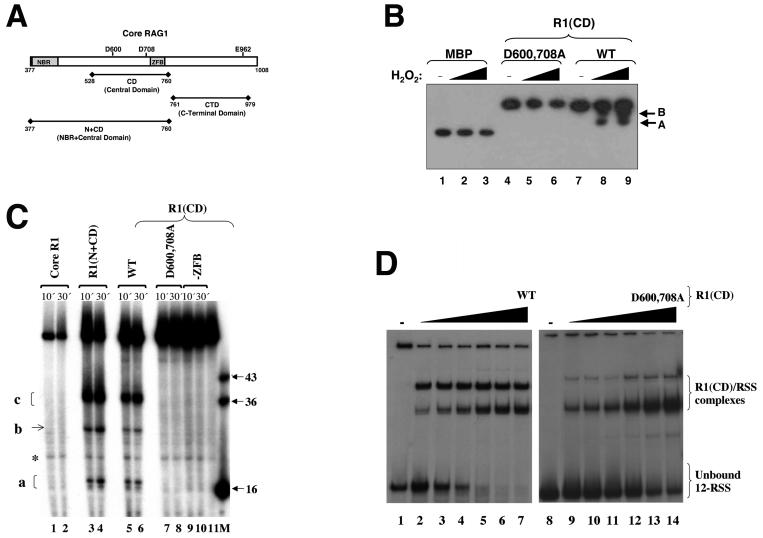
FIG. 1.
Metal-binding and ss DNA cleavage activities of the central domain in core RAG1. (A) Schematic of core RAG1. The positions of the three DDE active-site residues (D600, D708, and E962) are shown. The relative locations of the central domain, the C-terminal domain, and the NBR plus central domain are shown below the schematic of core RAG1. (B) Iron-induced hydroxyl radical peptide cleavage of the core RAG1 central domain. Lanes 1 to 3 contain MBP, lanes 4 to 6 contain the D600,708A R1(CD) double mutant, and lanes 7 to 9 contain the WT R1(CD). Both WT and mutant R1(CD) were fused to MBP. The reactions were initiated with the addition of either 0.02 or 0.0275% H2O2. The absence (−) of H2O2 from lanes 1, 4, and 7 is indicated. Western blotting with anti-MBP antibody revealed cleavage products (labeled A and B) in the reactions containing WT R1(CD). (C) ss DNA cleavage activity of the RAG1 domains. Cleavage reactions were performed using the ss WT 12-RSS (top) oligonucleotide substrate. The 5′-labeled substrate was incubated with core RAG1 (at 30 μg/ml) and the respective domains [the R1(CD) proteins at 30 μg/ml each and R1(N+CD) at 4 μg/ml] for the times indicated prior to electrophoresis. The major cleavage products are labeled a, b, and c. A band that is present with the substrate only is labeled with an asterisk. A RAG1 fragment that includes residues 528 to 721 is denoted as -ZFB. M indicates the marker lane with the lengths (in bases) of each marker indicated. Lane 11 contained only substrate. (D) Electrophoretic mobility shift assay demonstrating the interaction of WT and D600,708A R1(CD) with ss 12-RSS. The radiolabeled ss 12-RSS substrate was titrated with increasing concentrations of either WT or D600,708A R1(CD). The protein concentrations ranged from 0.2 to 0.8 μM [lanes 2 to 7 for WT and lanes 9 to 14 for D600,708A R1(CD)]. The samples were subjected to electrophoresis on a 6% nondenaturing polyacrylamide gel.