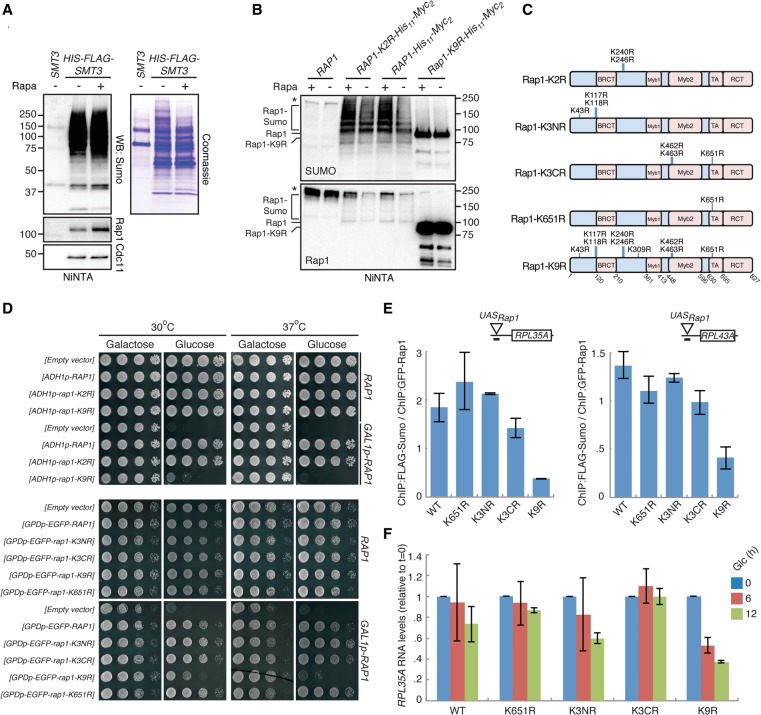
Figure 4.
Sumoylation of Rap1 is required for efficient RPG expression. (A) Sumoylation of Rap1 increases after rapamycin treatment. Cells were treated for 30 min with 100 nM rapamycin, and HIS6-FLAG-tagged Sumo was purified under denaturing conditions. Levels of Rap1 and Cdc11 in eluates were analyzed by Western blotting (left). Total protein levels in the eluates were visualized by Coomassie staining (right). (B) WT cells transformed with plasmids harboring HIS11-Myc2-tagged versions of RAP1, rap1-K2R, or rap1-K9R were treated with 100 nM rapamycin for 30 min. Rap1, Rap1-K2R, and Rap1-K9R were purified under denaturing conditions and visualized by Western blotting with Rap1 antibodies. (C) rap1 mutants used in this study. (D) Expression of rap1-K9R as the sole source of Rap1 results in severe growth defects. Cells expressing RAP1 from its endogenous promoter (“RAP1”) or from the GAL1/10 promoter (“GAL1_p-RAP1”) were transformed with an empty vector or with plasmids containing either RAP1, rap1-K2R, rap1-K9R, rap1-K3NR, rap1-K3CR, or rap1-K651R under control of the ADH1 or the GPD promoter, as indicated. Tenfold dilutions were spotted on plates containing either galactose or glucose and incubated for 2 d. (E,F) Rap1 sumoylation is important for transcription. FLAG-Sumo cells expressing endogenous RAP1 from the GAL1/10 promoter were transformed with plasmids harboring wild-type RAP1 (WT) or the rap1-K3NR, K3CR, K651R, or K9R alleles. Cells were grown to log phase in galactose, washed, and incubated in glucose for the indicated times, after which Sumo levels at the RPL35A and RPL43A promoters were analyzed by ChIP (E), and immature (i.e., unspliced) RNA levels of RPL35A were determined by qPCR (F). Error bars, SEM.