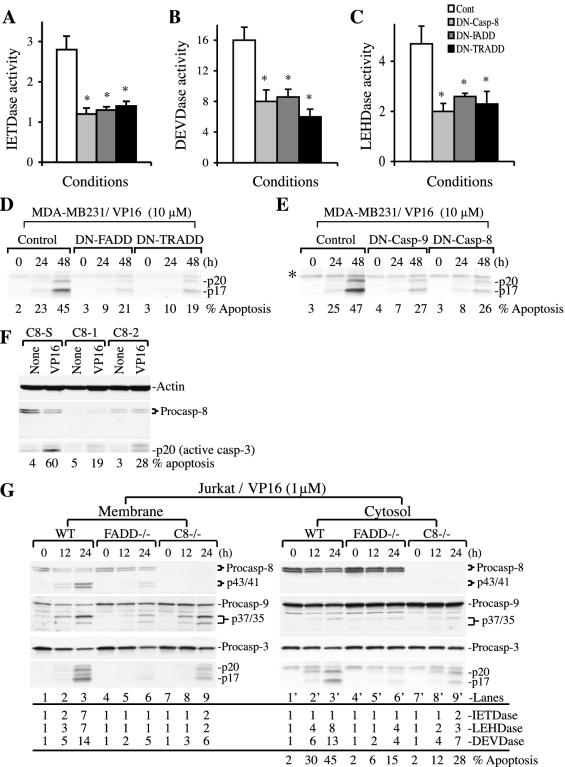
FIG. 6.
FADD/TRADD-dependent activation of caspase 8 and a critical role for caspase 8 in VP16-induced MDA-MB231 cell death. (A to C) DN-FADD and DN-TRADD inhibit caspase activation. Log-phase MDA-MB231 cells transfected with either DN-FADD or DN-TRADD were treated with VP16 (10 μM for 48 h). Whole-cell lysates were used to measure caspase activities as detailed in Materials and Methods. The transfection efficiency was 20 to 25%, as revealed by transfection of green fluorescent protein vectors. Data represent means ± standard deviations. *, P < 0.05 (Student's t test). (D) DN-FADD and DN-TRADD inhibit VP16-induced caspase 3 activation and apoptosis. Log-phase MDA-MB231 cells were transfected with DN-FADD or DN-TRADD. Twenty-four hours later, cells were treated with VP16 for 48 h. At the end, whole-cell lysates (60 μg/lane) were used in Western blotting of caspase 3. Apoptosis was quantified by DAPI staining. (E) DN caspase 9 or DN caspase 8 inhibits VP16-induced caspase 3 activation and apoptosis. Whole-cell lysates (60 μg/lane) prepared from MDA-MB231 cells treated for various conditions (as for panel D) were used in Western blotting of caspase 3. Apoptosis was quantified by DAPI staining. The asterisk indicates a nonspecific band. (F) LNCaP cells transfected with two siRNAs for caspase 8 (C8-1 and C8-2) or scrambled siRNA (C8-S). Twenty four hours after transfection, cells were treated with VP16 (10 μM for 48 h). At the end of treatment, apoptosis was quantified and whole-cell lysates (60 μg/lane) were used for Western blotting. (G) Wild-type, FADD−/−, or caspase 8−/− Jurkat cells treated with VP16 (1 μM) for 12 or 24 h were used to prepare membrane and cytosol fractions. Equal amounts of proteins (60 μg/lane) were used in Western blotting for the molecules indicated. Caspase activities were also determined by utilizing 50 μg of cytosol and membrane fractions. The percentages of apoptosis were determined as described in Materials and Methods. Cont, control; Procasp, procaspase; WT, wild type.