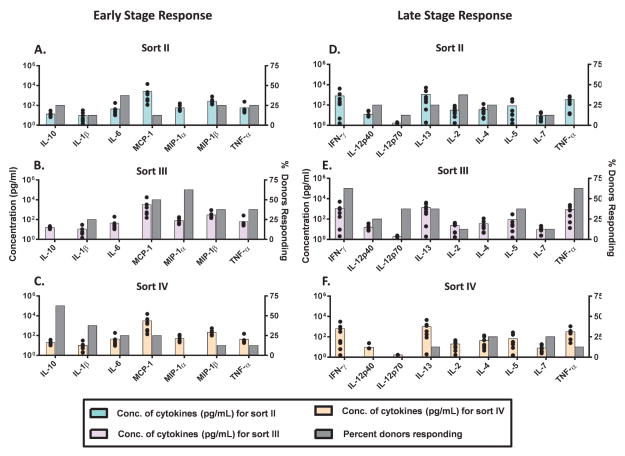
Figure 6.
Peripheral blood mononuclear cells (PBMC) from 8 human donors were incubated with FACS isolated mAb2 particle samples (containing very little soluble aggregates): sort II (enriched in 2–5 μm particles) (A, D), sort III (enriched for 5–10 μm particles) (B, E), and sort IV (enriched for particles > 10 μm) (C, F) and analyzed for their cytokine release. The FACS samples were added to the PBMC culture at similar particle numbers (low concentration; >9,000+ particles/mL in culture). These FACS samples as well as the relevant controls were tested for the release of signature cytokines by multiplex cytokine analysis at the early phase (20h) and late phase (7 days). The average concentration across donors (N=8, colored bars) of representative cytokines tested at the early and at the late phases is shown. The percentage of responding donors (gray bars) represents the number of the 8 donors tested that responded higher (by magnitude of secreted cytokines) to each sort. The black dots represent the concentration of cytokines secreted by each individual donor.