Table 2.
Substrate scope.a
| Entry | Substrate | Product | Yield [%]b |
|---|---|---|---|
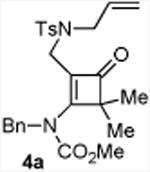
| 1 |
|
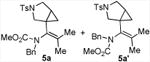
|
80 5a:5a′= 1:1d |
| 2 |
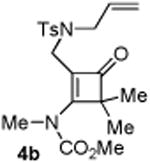
|
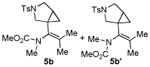
|
75 5b:5b′= 1:1d |
| 3 |
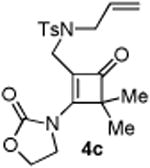
|
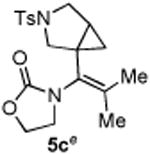
|
72 |
| 4 |
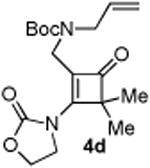
|
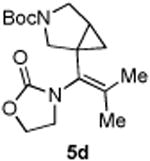
|
45 |
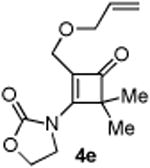
| 5 |
|
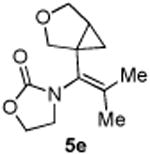
|
65 |
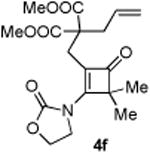
| 6 |
|
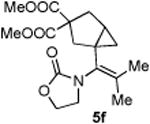
|
72 (86)c |
| 7 |
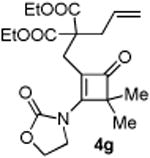
|
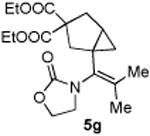
|
76 (90) |
| 8 |
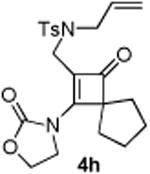
|
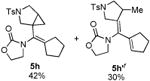
|
|
| 9 |
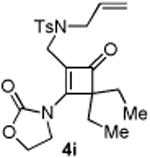
|
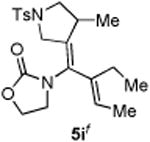
|
45 |
| 10 |
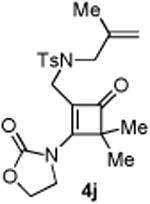
|
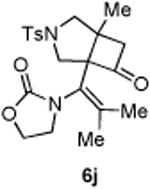
|
84 |
| 11 |
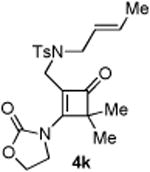
|
0 | |
| 12 |
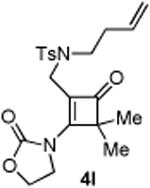
|
0 |
Each reaction was ran on a 0.1 mmol scale in a sealed 4 mL vial, using 5 mol% [Rh(CO)2Cl]2 and 12 mol% P(C6F5)3, in 1,4-dioxane (1.5 mL), at 130 °C, for 18 h.
Yields of isolated products.
The number in parentheses represents the yield based on recovered starting material (brsm).
The ratio was determined by 1H NMR.
The structure of 5c was confirmed by a series of 2D NMRs, DEPT, and HRMS.
5h′ and 5i were isolated as a single olefin-geometric isomer; the exact olefin geometry for each was not determined.
