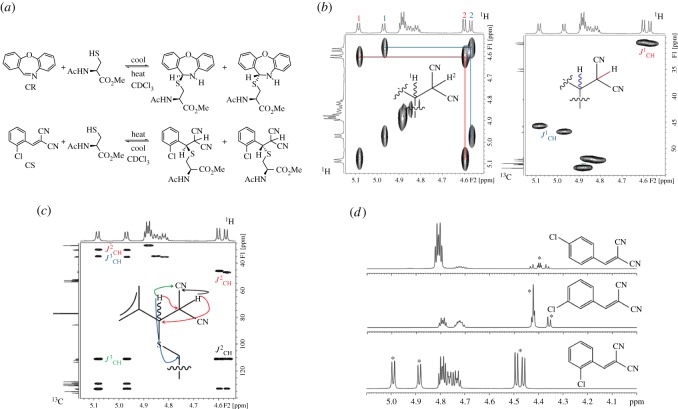
Figure 4.
CR and CS react reversibly with N-acetyl-l-cysteine methyl ester in CDCl3. (a) Equilibria and product diastereomers. (b) 1H COSY (left) and 1H-13C HSQC with DEPT-135 editing (right) NMR spectra: the CH2 group of the diastereomers gives a negative signal. (c) 1H-13C HMBC NMR spectrum showing signals for the two diastereomers. (d) 1H NMR spectra of mixtures of 2- (CS), 3- and 4-Cl analogues with the cysteine ester. The substituent has a strong electronic effect on the coupling pattern of the protons of the SCHCH(CN)2 group of the product (marked with asterisks). A Cl atom in the 2-position produces symmetrical doublets for both diastereomers. A Cl atom in the 3-position causes these signals to overlap, but gives an integral of 3:1 consistent with the proposed structures. A Cl atom in the 4-position produces four doublets, but due to their similarity, roofing effects are observed. These types of coupling patterns were seen across all the substituents and positions studied.