Table 2.
Physiological action on male human volunteers of four BMNs dispersed in a 100 m3 chamber by spraying in benzene or acetonea.
| compoundb | concentration | physiological symptoms | conclusion |
|---|---|---|---|
2-Cl (CS) [3]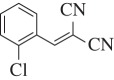
|
0.01 ppm (benzene) | detected immediately by ‘peppery’ irritation of the upper respiratory passages, the throat and chest were involved within 15 s, and lachrymation rapidly followed. ‘Prickling’ effect on skin was noted. This concentration was considered about the limit of tolerability for 1 min exposure | 2-Cl is a lachrymator and sternutator. It is immediately intolerable in a concentration of 0.2 ppm, and in a concentration as low as 0.01 ppm causes eye irritation, lachrymation and respiratory irritation sufficient to render work impossible if there is no protection. The irritant symptoms subside rapidly on leaving the chamber |
| 0.2 ppm (benzene) | this concentration was immediately intolerable by reason of ‘peppery’ irritation of the whole respiratory tract, profuse lachrymation and ‘prickling’ effect on exposed skin | ||
2-NO2[42]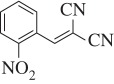
|
0.01 ppm (benzene) | immediate and severe irritation of the whole respiratory tract with much coughing followed by ‘prickling’ of the skin particularly where it was moist, and irritation of the eyes with lachrymation. The limit of tolerability was reached in 35 s and subjects left the chamber | 2-NO2 is a powerful sternutator and lachrymator at a nominal concentration of 0.01 ppm. At a nominal concentration of 0.002 ppm, it produces physiological symptoms which, though not intolerable, would seriously interfere with the performance of duties. Prickling of the skin, particularly where it is moist is a notable symptom. The physiological symptoms rapidly subside after leaving the experimental atmosphere |
| 0.002 ppm (benzene) | the whole respiratory tract was involved within 1 min. The irritation which was of the ‘hot peppery’ type induced coughing and a desire to sneeze. Eye irritation developed and also slight ‘prickling’ of the skin. The exposure was limited to 10 min and though not intolerable, this concentration was decidedly uncomfortable and would seriously interfere with work. The eye irritation did not progress to lachrymation | ||
2-Br [43]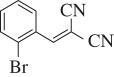
|
0.01 ppm (acetone) | rapid onset of irritation of the eyes, nose, throat and in some cases the chest all within 15 s. Three out of five subjects left the chamber in 60 s, 90 s and 210 s, respectively. The other two subjects remained in for 10 min and reported that the symptoms tended to subside during exposure. Throat and chest irritation was not marked. Prickling of the skin, particularly where moist, was noted | 2-Br possesses lachrymatory and sternutatory properties. In addition, ‘prickling’ of the skin particularly over moist areas is a feature with this compound. All of the symptoms rapidly subsided after leaving the chamber |
| 0.2 ppm (acetone) (men equipped with respirators) | the object of this exposure was to observe the irritant effects on exposed skin in a higher concentration. Protected with gas mask and with the exposed skin at normal (cool) temperature, ‘prickling’ was not marked and somewhat slow in developing. One subject removed his respirator and found the concentration quite irrespirable. This was followed by marked prickling of the skin on the moist areas of face, and on lachrymation marked stinging of the skin along the course of tears on the face | ||
2-H (BMN) [44]
|
0.2 ppm (benzene) | detected immediately by irritation of the upper respiratory passages. The irritation was of the ‘peppery’ type. The chest became affected within 1 min causing mild coughing and restricted breathing. There was also tear formation but no actual lachrymation. Although this concentration would interfere with carrying out ordinary duties, the symptoms could not be described as indicating the limit of tolerability during an exposure of 3 min. Towards the end of this exposure, the discomfort was subsiding | BMN is an irritant which attacks the respiratory tract and to a lesser degree the eyes. In both respects, however, it is far less potent than many other well-known compounds |
bThe BMNs used in the historic study summarized in this table were prepared by the method in the original paper of Corson and Stoughton (from whose initials CS derives its codename) [2]. The 2-NO2 and 2-Br analogues are not described in that paper. The former was obtained at Porton Down after two recrystallizations from ethanol as salmon pink needles (mp 141°C) and the latter similarly as cream crystals (mp 90–91°C). The four BMNs examined in the historic trial were analytically pure.
