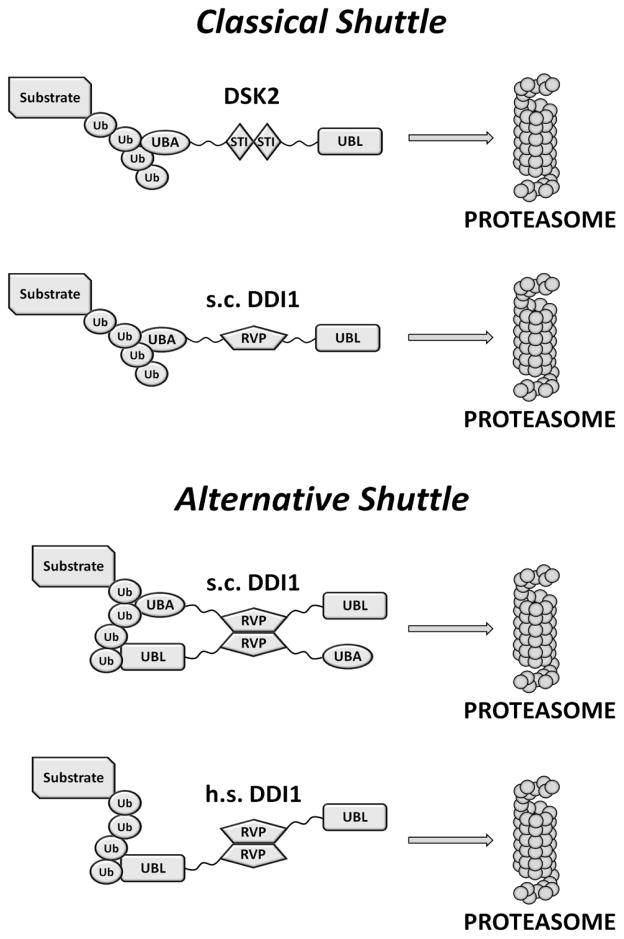
Figure 9.
Schematic representation of a possible function of Ddi1 as a proteasomal shuttle in yeast (s.c.) and humans (h.s.). A “classical” shuttle protein (e.g. Dsk2) employs a UBA domain to recognize and bind (poly)Ub tag on a substrate protein and a UBL domain to target it to the proteasome (e.g.,(Zhang et al., 2009)). In yeast Ddi1, both the UBA and UBL domains can recognize polyubiquitinated substrates. Human Ddi1 lost its UBA domain during the evolution but still contains the UBL domain; the dual functionality of the UBL domain should allow Ddi1 to both bind polyUb tag and deliver polyubiquitinated substrates to the 26S proteasome for degradation.