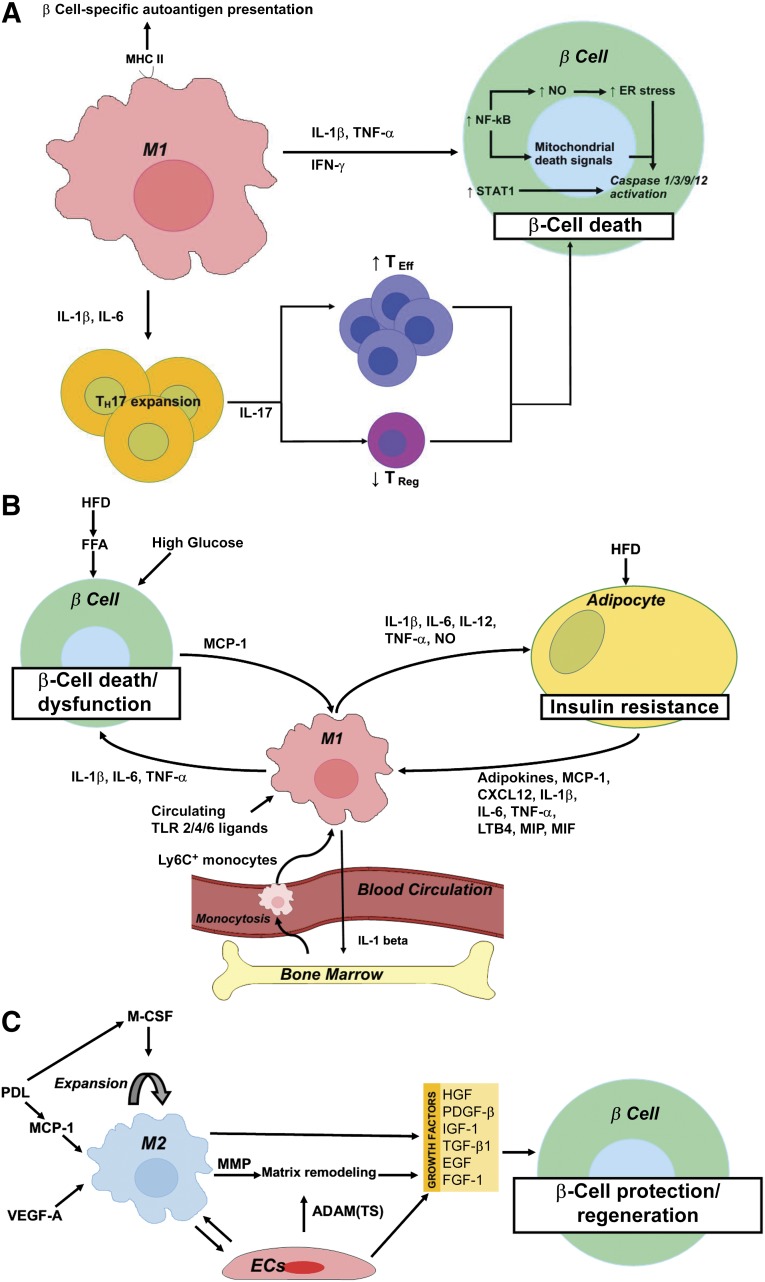
Figure 3.
Schematic representation summarizing the role of macrophages in the pathogenesis of type 1 diabetes mellitus (A), type 2 diabetes mellitus (T2D) (B), and during β-cell protection and regeneration (C). (A): M1 macrophages contribute to β-cell death through (a) MHC II-mediated presentation of β-cell-specific autoantigens, (b) IL-1β and IL-6-mediated TH17 expansion and subsequent IL-17-mediated TEff/TReg imbalance, and (c) a direct cytotoxic effect of IL-1β and/or TNF-α plus IFN-γ with downstream activation of NF-κB and STAT1. NF-κB activation results in NO production and increased ER stress and cytochrome c release from mitochondria, the latter acting as a mitochondrial death signal. Ultimately, NF-κB and STAT1 activation results in caspase 1/3/9/12 activation and subsequent β-cell death. (B): High circulating glucose and FFAs contribute to β-cell death in T2D. In addition, a HFD and circulating FFAs promote MCP-1 secretion from β cells and subsequent intraislet accumulation of M1-like macrophages. Moreover, a HFD and onset of T2D correlate with elevated circulating levels of TLR2 and -4 ligands, which stimulate the secretion of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) from these recruited macrophages, thereby further contributing to β-cell death and dysfunction. A high-fat diet and lipid accumulation also promote adipo- (leptin, resistin), chemo-, and cytokine (IL-1β, IL-6, TNF-α, MCP-1, LTB4, CXCL12, MIP, and MIF) secretion from adipocytes, thereby promoting the recruitment to and activation of Ly6C+ monocytes and M1 macrophages in adipose tissue. These exert detrimental effects on peripheral insulin signaling through secretion of IL-1β, IL-6, IL-12, and TNF-α, and production of NO. Finally, M1 adipose tissue macrophages promote myelopoiesis and monocytosis through IL-1β secretion, thereby further enhancing macrophage accumulation in inflamed adipose tissue and, thus, establishing a positive feedback loop in T2D pathogenesis. (C): M2 macrophages accumulate in the pancreas after transgenic, β cell-specific VEGF-A overexpression or partial PDL, in the latter via MCP-1-mediated recruitment and M-CSF-dependent proliferation of macrophages. M2 macrophages promote β-cell protection and regeneration through secretion of several growth factors (i.e., TGF-β1, EGF, PDGF-β, IGF-1) in concert with endothelial cell-derived growth factors (i.e., HGF, FGF-1, IGF-1, TGF-β1, and PDGF-β). M2 macrophages and endothelial cells moreover produce matrix remodeling factors—MMP (i.e., MMP12, MMP13) and ADAM and ADAM(TS) (i.e., ADAM12, ADAMTS9), respectively—facilitating growth factor bioavailability. Abbreviations: ADAM, a disintegrin and metalloproteinase; ADAM(TS), a disintegrin and metalloproteinase with thrombospondin motifs; CXCL, chemokine (C-X-C motif) ligand; EGF, epidermal growth factor; ER, endoplasmic reticulum; FFA, free fatty acid; FGF, fibroblast growth factor; HFD, high-fat diet; IGF, insulin-like growth factor; IL, interleukin; LTB, leukotriene B; MCP, monocyte chemoattractant protein; M-CSF, macrophage colony-stimulating factor; MIF, macrophage migration inhibitory factor; MIP, macrophage inflammatory protein; MMP, matrix metalloproteinase; NF-κB, nuclear factor κB; NO, nitric oxide; PDGF, platelet-derived growth factor; PDL, pancreatic duct ligation; TEff, T effector; TH, T helper; TReg, T regulator; TLR, Toll-like receptor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.