Scheme 3.
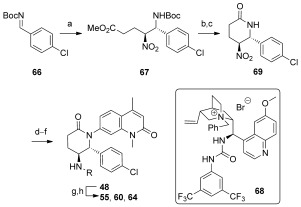
Organocatalytic enantioselective synthesis of BRD9 inhibitors. Reagents and conditions: a) 11, K2CO3, 68 (10 mol %), TBME, −20 °C, 70 %, d.r. 7:1, eemajor 90 %/eeminor 90 %; b) TFA, CH2Cl2; c) DBU, CH2Cl2, 73 % (2 steps); d) NiCl2⋅6 H2O, NaBH4, MeOH, 0 °C; e) Boc2O, 74 % (2 steps); f) 4, K3PO4, CuI, (±)-trans-1,2-diaminocyclohexane, 1,4-dioxane, 97 °C, 65 %; g) HCl/dioxane, 96 %; h) RCl, TEA, CH2Cl2 or RNCO, CH2Cl2, 25–40 %. DBU=1,8-diazabicyclo[5.4.0]undec-7-ene, TBE=tert-butyl methyl ether, TEA=triethylamine.
