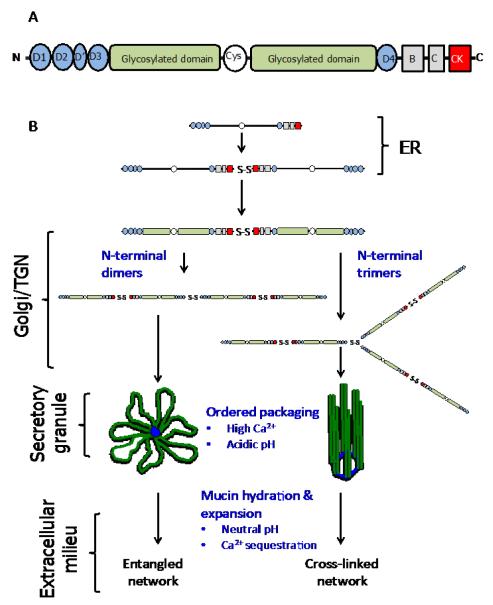
Fig. 2. Cartoon of mucin structure and assembly.
(A) Polymeric, gel-forming mucins all share a common structural architecture. The N- and C-terminal domains have a high cysteine content and these form intra- and intermolecular disulphide bonds. The central highly-glycosylated domains (mucin domains) are enriched in serine, threonine and proline residues and the O-glycans are covalently attached, via the linkage sugar N-acetylgalactosamine, to serine and threonine residues. The O-glycan chains are comprised of N-acetylglucosamine, galactose, N-acetylgalactosamine, fucose and sialic acid; galactose can be modified by sulphation. The number, length and amino acid sequence of these glycosylated domains differ between mucins. The glycosylated domains are interrupted with cys-domains, and the number of these cysteine-rich regions differs between mucins. For more detailed reviews on the primary structure of MUC2, MUC5AC and MUC5B the reader is referred to the following articles - Dekker et al., 2002 and Rose and Voynow, 2006. (B) The early steps in polymeric mucin assembly are well accepted. In the endoplasmic reticulum (ER), the non-O-glycosylated polypeptide forms dimers via disulphide bonds formed between the CK-domains. In the golgi/trans-golgi network (TGN), mucin dimers are O-glycosylated and then multimerise by disulphide bonds formed between N-terminal D3 domains. There are two mechanisms proposed for this step, multimers form from dimers of dimers (MUC5B, Ridley et al. , 2011, Round et al. , 2004, Sheehan et al. , 2004, Thornton et al. , 1990) or trimers of dimers (MUC2, Ambort et al., 2012). The mucin multimers are then packged in an ordered state within secretory granules. The acidic pH inside the granules and their high Ca2+ content facilitates mucin organisation via non-covalent interactions between mucin N-termini (Ambort et al., 2012). After secretion mucins hydrate and expand to form mucus.