Figure 3.
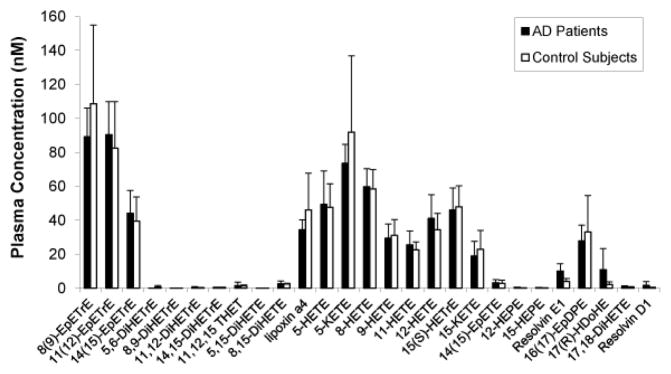
Oxylipin composition of plasma samples from Alzheimer’s (AD) patients and control subjects. Whole blood samples were obtained at fasting, after which the plasma fractions were isolated and analyzed by liquid chromatography-tandem mass spectrometry for the following analytes: 8(9)-epoxy-8Z,11Z,14Z-eicosatrienoic acid (EpETrE), 11(12)-EpETrE, 14(15)-EpETrE, 5,6-dihydroxy- 5Z, 8Z, 11Z- eicosatrienoic acid (DiHETrE), 8,9-DiHETrE, 11,12-DiHETrE, 14,15-DiHETrE, 11,12,15 trihydroxyeicosatrienoic acid (THET), 5,15-dihydroxy-5Z,9E,11Z, 13E-eicosatetraenoic acid (DiHETE), 8,15-DiHETE, lipoxin a4, 5-hydroxyeicosatetraenoic acid (HETE), 5- 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid (KETE), 8-HETE, 9-HETE, 11-HETE, 12-HETE, 15(S)-hydroxy-8Z,11Z,13E-eicosatrienoic acid (HETrE), 15-KETE, 14(15)-epoxy- 5Z, 8Z, 11Z, 17Z- eicosatetraenoic acid (EpETE), 12-hydroxy-5Z,8Z,10E,14Z,17Z-eicosapentaenoic acid (HEPE), 15-HEPE, Resolvin E1, 16(17)-epoxy-4Z,7Z,10Z,13Z,19Z-docosapentaenoic acid (EpDPE), 17(R)-hydroxy-4Z,7Z,10Z,13Z, 15E,19Z-docosahexaenoic acid (HDoHE), 17,18-dihydroxy-5Z,8Z, 11Z,14Z-eicosatetraenoic acid (DiHETE), and Resolvin D1. Data are expressed as means ± SD (n=5 AD patients, and 5 control subjects).
