Fig 7. Oligomerization of TMEM120A and B.
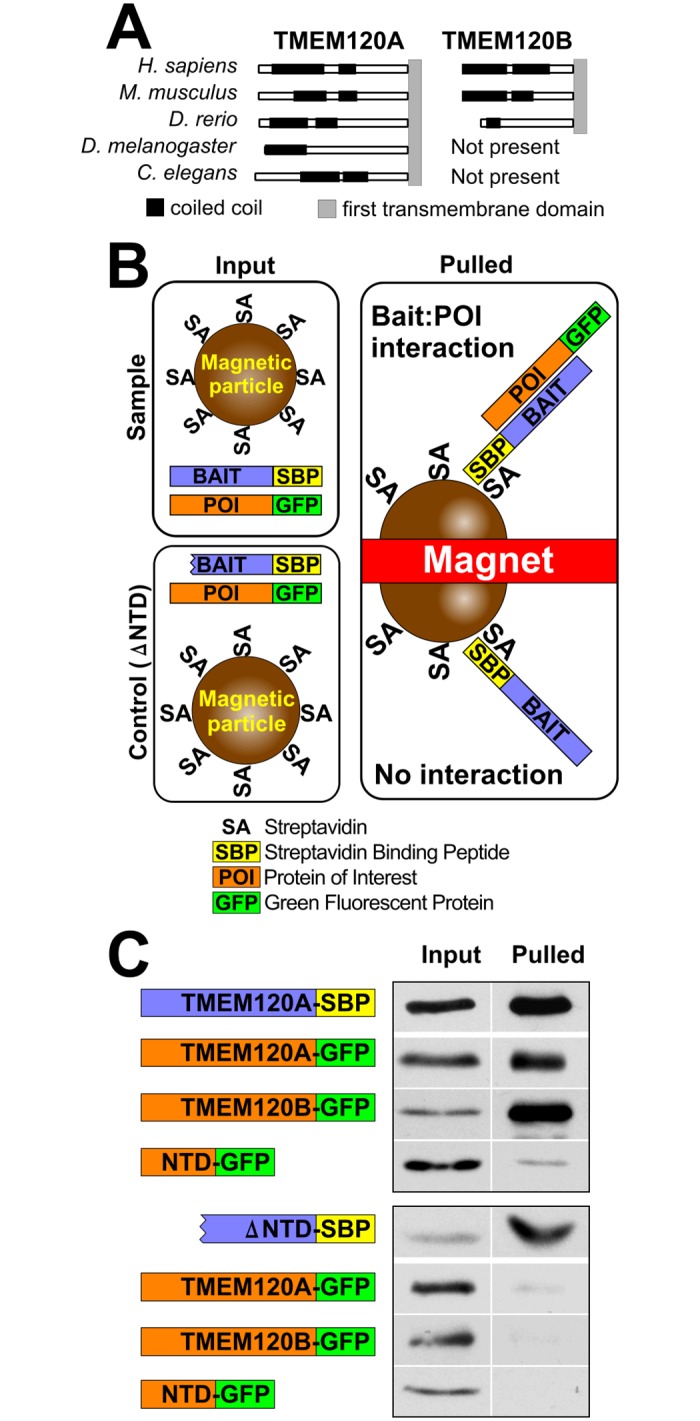
(A) Coiled-coil domains are strongly predicted within the N-terminal regions of both TMEM120A and B using the COILS2 server for all organisms having both TMEM120 genes and predicted in the single protein for organisms having only one TMEM120 gene. (B) Schematic of assay and expected outcomes. Cells co-express streptavidin binding protein (SBP) fused to one TMEM120 protein and GFP fused to the same or another TMEM120 protein. The cells are lysed and the SBP fusion proteins are pulled from the lysate with streptavidin conjugated to magnetic beads. If the other TMEM120 protein fused to GFP is pulled out together with the SBP tagged protein it indicates interaction between the two TMEM120 proteins expressed. As a negative sontrol, TMEM120A-SBP fusion lacking predicted coil-coiled domains (deleted 2–105 amino acids, ΔNTD) was used. (C) Proteins pulled out of the lysates by the magnetic streptavidin coated beads were analyzed by Western blot using antibodies to GFP to detect the GFP fusions and streptavidin to test the SBP fusions. This confirmed both hetero- and homo-dimer formation are possible among TMEM120 proteins. The soluble N-terminal fragment containing the predicted coiled coils also interacted with the full-length protein, but not the ΔNTD mutant.
