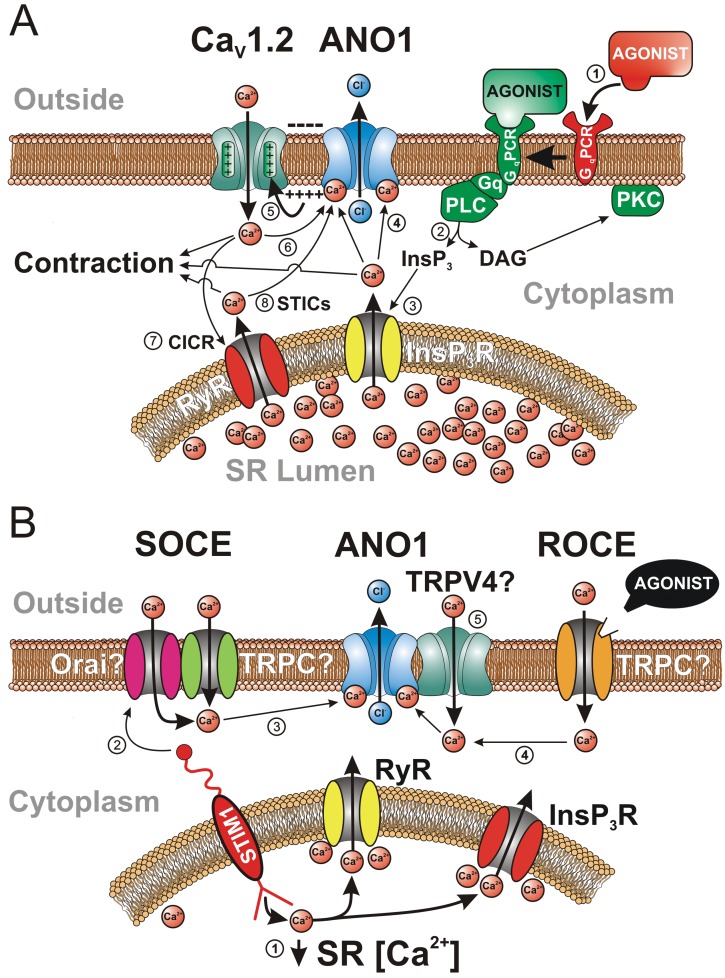
Figure 5.
Various sources of Ca2+ activating the Ca2+-activated Cl− channel (CaCC) ANO1 in vascular smooth muscle cells. A, The classical mode of activation of CaCCs by stimulation of a Gq-coupled receptor (GqPCR) with a constricting agonist (e.g., 5-hydroxytryptamine, angiotensin II, endothelin), leading to an elevation of intracellular Ca2+ concentration and contraction, is illustrated in this panel by the circled numbers: (1) binding of an agonist to a GqPCR; (2) breakdown of membrane-bound phosphatidyl-inositol by Gq-stimulated phospholipase C (PLC), leading to the production of the second messengers inositol trisphosphate (InsP3) and diacylglycerol (DAG); (3) while DAG stimulates protein kinase C (PKC), which targets many proteins involved in contraction and cell proliferation, InsP3 diffuses toward the sarcoplasmic reticulum (SR), where it binds to and activates a Ca2+-permeable receptor-operated channel (InsP3R), triggering Ca2+ release in the cytoplasm; (4) Ca2+ diffuses in the cytoplasm and activates contraction and ANO1; (5) opening of ANO1 leads to Cl− efflux and membrane depolarization due to an outwardly directed electrochemical gradient for Cl−, which stimulates voltage-gated L-type Ca2+ channels encoded by the gene CaV1.2; (6) Ca2+ entry through CaV1.2 would in turn promote actomyosin bridge cycling and further stimulate ANO1, establishing a positive-feedback loop sustaining membrane depolarization and Ca2+ entry; (7) there is evidence for stimulation of ANO1 in some vascular myocytes by Ca2+ entry through CaV1.2 triggering SR Ca2+ release from ryanodine receptors (RyR) in a process called Ca2+-induced Ca2+ release (CICR), which can provide an additional stimulus for ANO1 activation by CaV1.2; (8) spontaneous Ca2+ release by RyR in the SR, giving rise to “spontaneous transient inward currents,” or STICs, produced by transient openings of ANO1. B, Activation of ANO1 by voltage-independent Ca2+ entry pathways: (1) Ca2+ unbinding from the SR Ca2+ sensor protein STIM1 (stromal interacting molecule 1) by a decrease in the concentration of Ca2+ in the SR due to spontaneous Ca2+ leakage or mediated by an agonist leads to clustering of portions of the SR with the plasma membrane; (2) STIM1 then physically interacts with a complex composed of one or more members of the canonical transient receptor potential (TRPC) and/or Orai families of cation channels to trigger store-operated Ca2+ entry (SOCE); (3) Ca2+ entry via SOCE can then stimulate ANO1; (4) ANO1 can also be stimulated by Ca2+ influx through a receptor-operated channel stimulated by an agonist (ROCE); (5) although hypothetical, it is possible that ANO1 could be stimulated locally by Ca2+ influx through TRPV4 (vanilloid transient receptor potential) channels physically interacting with the anion channel.