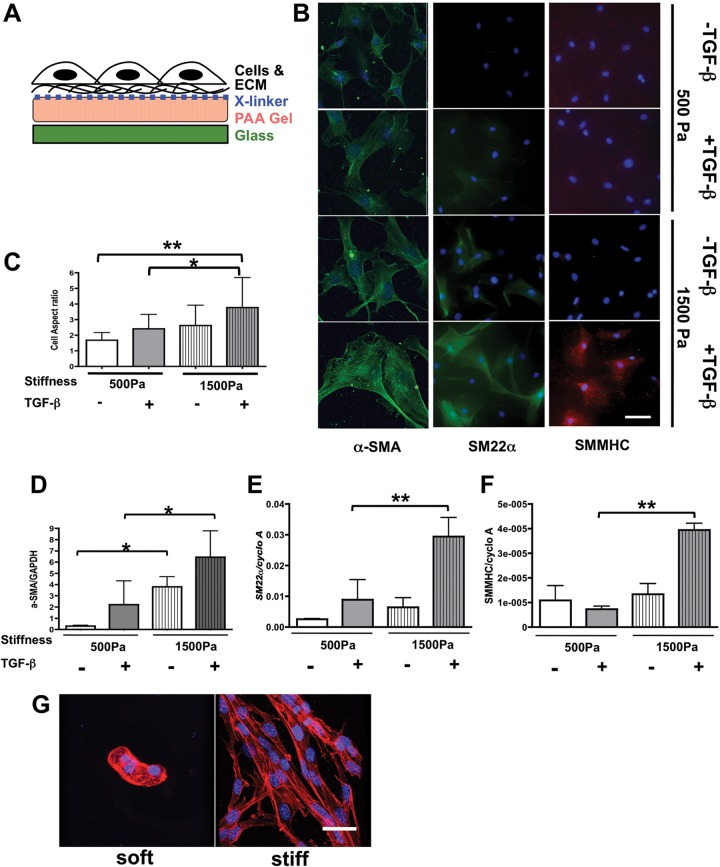
Figure 7.
Transforming growth factor (TGF)–β–dependent 10T1/2 cell differentiation tightly interacts with Prx1-dependent extracellular matrix (ECM) stiffness. A, Schema for the stiffness-controlled polyacrylamide (PAA) gel 2-D culture system. B, 10T1/2 cells were cultured on PAA gel 2-D substrates with a stiffness of either 500 (top) or 1,500 (bottom) Pa, which is parallel to the E17.5 Prx1-lung stiffness, in the presence or absence of TGF-β stimulation. Smooth muscle cell differentiation was evaluated by expression of α–smooth muscle actin (SMA; left, green), SM22α (middle, green), and smooth muscle myosin heavy chain (SMMHC; right, red). 4′,6‐Diamidino‐2‐phenylindole dihydrochloride (blue) was used for nuclear staining. Scale bar = 10 μm. C, Aspect ratio of cells cultured on 2-D PAA gels under the above-described conditions was examined to quantify cell morphology change. D–F, Real-time polymerase chain reaction results for 10T1/2 cells cultured on 2-D PAA gels of either 500- or 1,500-Pa stiffness with or without TGF-β stimulation. α-SMA (D), SM22α (E), and SMMHC (F) messenger RNA expression was examined and normalized by GAPDH or cyclophilin A (cyclo A) expression. One asterisk indicates P < 0.05, and two asterisks indicate P < 0.01. G, 3-D projection of confocal images of F-actin-stained 10T1/2 cells cultured in soft and stiff 3-D methacrylated hyaluronic acid synthetic scaffolds. F-actin is red, nucleus is blue. Scale bar = 20 μm.