Abstract
Introduction: The objective of this study was to examine the prognostic value of Ki-67 expression in conversion therapy for colorectal liver-confined metastases. Methods: We enrolled a total of 96 patients including 54 patients who received oxaliplatin- or irinotecan-based chemotherapy and curative hepatectomy for initially unresectable metastases (conversion group) and 42 patients with initially resectable liver metastases (straight hepatectomy group). Ki-67 expression was examined in 96 resected specimens but excluded the 2 specimens that revealed no residual cancer cells in conversion group. Results: Conversion therapy leads to greater survival that is equivalent to that straight hepatectomy group. In conversion group, high Ki-67 expression (> 30%) levels were detectable in 33 patients (64%) after chemotherapy prior to conversion therapy. High Ki-67 expression was significantly associated with shorter disease-free survival and worse overall survival (P < 0.01 in both), and was an independent worse prognostic factor of disease-free survival and overall survival (hazard ratio [HR] and P-values were 5.608, 0.001 and 5.366, 0.04, respectively) in patients with conversion therapy. Interestingly, even in the patients with RECIST PR (n = 32), high Ki-67 expression was significantly shorter disease-free survival compared to low Ki-67 expression (P < 0.001). In contrast to conversion group, there was no significant difference in disease free survival and overall survival between low (n = 14, 33%) and high (n = 28, 67%) Ki-67 expressions in patients with straight hepatectomy (P = 0.14 and 0.74, respectively). Conclusions: Residual Ki-67 expression is a useful biomarker for worse prognostic outcomes after conversion therapy. High Ki-67 expression may be a biomarker of micrometastases containing aggressive cancer cells.
Keywords: Colorectal cancer, liver metastasis, conversion therapy, Ki-67 index
Introduction
Colorectal cancer (CRC) is the second most common malignant neoplasia worldwide. Liver metastasis, or stage IV cancer, is the most common status of colorectal metastasis patients. Approximately 50% of colon cancer patients will be diagnosed with hepatic metastases either at the time of initial presentation or as a result of disease recurrence [1]. The latest developments of irinotecan- or oxaliplatin-based systemic chemotherapies with or without molecularly targeted agents have dramatically improved the tumor response rates and prognostic outcomes in patients with liver metastases from CRC [2-6]. However, surgical resection remains the only potentially curative therapeutic option for patients with liver metastasis from CRC [7,8]. Highly effective chemotherapy regimens enable complete hepatic resection for initially unresectable liver metastasis from CRC, which is termed conversion therapy. Conversion therapy after recent oxaliplatin- or irinotecan-based systemic chemotherapy with molecular targets for initially unresectable liver metastasis has been achieved in approximately 12-49% of patients [5,9-11]. Thus, conversion therapy can produce favorable prognostic outcomes relative to patients with unresectable CRC liver metastases. Although conversion therapy is successful, disease relapse is high and occurs in approximately two-thirds of patients [1]. These findings emphasize that most patients still harbor minimal but biologically active micrometastases after effective chemotherapy and conversion therapy. Therefore, adjuvant systemic treatment is recommended to improve life expectancy [12]. However, many randomized clinical trials of adjuvant chemotherapy after curative hepatectomy for liver metastasis (Stage IV) from CRC have failed to demonstrate any overall survival advantage [13-16]. In addition to the need for more effective anticancer drugs, these unfavorable results suggest that the trials that were conducted included patients who did not obtain survival benefits from adjuvant treatment because they lacked biologically active micrometastases. Thus, the identification of patients with a high risk of disease recurrence after conversion therapy might lead to better selection of patients who would benefit from adjuvant chemotherapy based on the principles of personalized medicine [17,18].
Ki-67 is a nuclear non-histone protein that has been reported to identify high-grade tumors in several cancers [19-22]. Based on previous findings, we assessed whether Ki-67 expression levels in resected liver metastases from patients undergoing conversion therapy predicted worse prognostic outcomes after conversion therapy and explored the role of Ki-67 as a biomarker.
Patients and methods
The study was designed to retrospectively assess the prognostic value of Ki-67 expression levels in patients with conversion therapy after oxaliplatin- or irinotecan-based systemic chemotherapy for liver-confined metastases from CRC. Patients were enrolled in this study based on the following inclusion criteria: (1) initially unresectable or marginally unresectable colorectal metastases confined to the liver as assessed during radiological imaging assessments; and (2) oxaliplatin- or irinotecan-based systemic chemotherapy followed by conversion therapy. The patients who received systemic chemotherapy prior to conversion therapy in the other institute were excluded because of unclear judgment of initially resectable or unresectable liver metastases. Between January 2005 and March 2013, 261 consecutive patients had been treated for colorectal liver metastases in the Department of Gastroenterological Surgery, Kumamoto University. Among them, 145 patients were treated for liver-confined metastases (Figure 1A), of which 42 patients were eligible for curative hepatectomy (initially resectable cases) (a mean follow-up of 43 months after surgery). Two patients underwent local ablation therapy. Treatment with best supportive care was chosen for six patients. The remaining 95 patients were treated with systemic chemotherapy. Eighty-six of 95 patients were treated with oxaliplatin- or irinotecan-based chemotherapy with or without molecular targets. Among the remaining 9 patients, seven were treated with other regimens (hepatic arterial infusion in four patients, an oral fluoropyrimidine [S-1] in one patient, and S-1/leucovorin in two patients) and the others (two patients) were excluded from this study because of the systemic chemotherapy in the other institute. Fifty-four patients who had been successfully treated with conversion therapy were eligible for the present study (conversion group). In twelve of these 54 patients, elective local ablation therapy was simultaneously used for unresectable liver metastases because of the small size and deep location of the tumor during conversion therapy [23]. The remaining 32 patients could not achieve conversion therapy because of ineffective chemotherapy (unresectable cases). Most patients receiving conversion therapy received adjuvant chemotherapy except the patient who died postoperatively. After a mean follow-up of 33 months after surgery, 38 of 54 patients with conversion therapy were alive (16 with no evidence of disease, 22 alive with disease recurrence), and 14 had died of disease recurrence. Two patients died of other disease within 1 year. The characteristics of these patients are listed in Table 1. Two of the 54 patients revealed no detectable residual cancer cells by hematoxylin and eosin staining in the resected liver specimens and therefore were not eligible for Ki-67 staining. This study was approved by the Human Ethics Review Committee of the Graduate School of Medicine, Kumamoto University (Kumamoto, Japan). Written informed consent regarding the use of biological specimens for investigational purposes was obtained from all patients.
Figure 1.
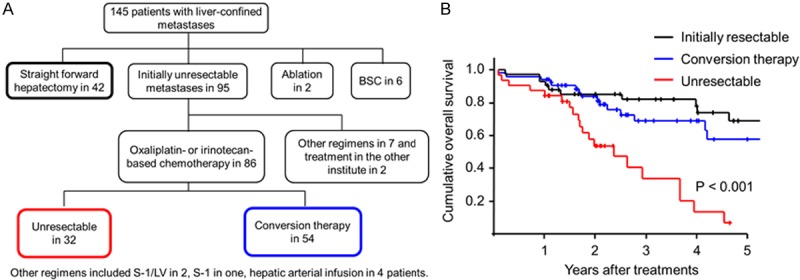
Comparison of prognostic outcomes among patients with initially resectable, conversion therapy for initially unresectable, and unresectable liver metastases. A. A flow chart of patients with initially resectable, conversion therapy for initially unresectable, and unresectable liver metastases. B. A Kaplan-Meier survival analyses of the overall survival in patients with initially resectable, conversion therapy for initially unresectable, and unresectable liver metastases. The log-rank test was used.
Table 1.
Baseline characteristics of 54 patients who received conversion therapy
| Age median (range), yr | 65 (35-83) |
| Male, n (%) | 31 (58%) |
| Platelet count, median (range), 104/μL | 19.1 (6.5-37.5) |
| Total bilirubin, median (range) mg/dL | 0.7 (0.3-1.3) |
| Serum albumin, median (range), g/L | 3.9 (3.0-4.4) |
| Prothrombin activity, median (range), % | 108 (54-137) |
| Serum CEA level, median (range), ng/mL | 6.1 (1.0-172.6) |
| Size of largest tumor, median (range), mm | 25.5 (5-160) |
| Tumor number, median (range) | 2.5 (1-19) |
| Synchronous/Metachronous | 34/19 |
| Molecular targets (present), n (%) | 27 (50%) |
| Chemotherapy cycles, median (range) | 6 (2-38) |
| RECIST CR/PR/SD/PD | 2/32/15/5 |
CEA, carcinoembryonic antigen; RECIST, response evaluation criteria in solid tumors (version 1.1).
Immunohistochemical staining
The sample processing and immunohistochemical procedures were performed as described in a previous report [24]. Formalin-fixed, paraffin-embedded blocks of colorectal liver metastases were cut into 3-µm sections. The sections were autoclave-pretreated in Histofine antigen retrieval solution (pH 9) (Nichirei). The endogenous tissue peroxidase activity was blocked using 3% hydrogen peroxide, and the sections were then incubated with diluted primary antibodies. A subsequent reaction was performed with a biotin-free horseradish peroxidase enzyme-labeled polymer from the Envision Plus detection system (Dako Co.). A positive reaction was visualized with the addition of diaminobenzidine solution, which was followed by counterstaining with Mayer’s hematoxylin. Primary mouse monoclonal antibodies (mAbs) against Ki-67 (1:100 dilution; Dako Cytomation, Glostrup, Denmark) were used for this study. Two investigators (N. Y. and T. H.) independently scored all of the immunohistochemical staining results. Five random visual high-power fields per lesion were evaluated to determine the highest number of Ki-67-positive nuclei. In cases with multiple tumors, at least 2 or 3 tumors were chosen in order of size to evaluate and determine Ki-67 expression levels. The samples were divided into Ki-67-positive expression (> 30%) and Ki-67-negative expression (≤ 30%) groups.
Statistical analyses
Statistical analyses were performed using a commercial statistical software package (SPSS for Windows, version 11.0; SPSS, Inc., Chicago, IL) as previously [25]. Continuous values were evaluated using the Mann-Whitney U-test. Categorical variables were compared using the χ2 test. Overall survival and disease-free survival were calculated using the Kaplan-Meier method and were compared using a log-rank test. Univariate and multivariate Cox regression analyses were used to examine the relationship between clinico-pathological factors at hepatectomy and prognostic outcomes. A P-value of less than 0.05 (two-tailed t-test) was considered statistically significant.
Results
Conversion therapy improved the prognostic outcomes compared to patients with unresectable liver metastases, but high relapse still occurred within 1 year after conversion therapy
The treatment with conversion therapy lead to greater survival that was equivalent to patients diagnosed with initially resectable liver metastases based on previous reports [26,27] (Figure 1B). The disease relapse rate in patients with conversion therapy was higher, particularly within 1 year after hepatectomy, than in patients with initially resectable liver metastases [54% (29/54) vs. 36% (15/42), respectively]. These findings suggested that residual micrometastases in the remnant liver were present after effective chemotherapy.
Assessment of cell proliferative activity in residual cancer cells using Ki-67 immunostaining and evaluation of its clinical significance
We performed immunohistochemical staining for Ki-67 in the resected liver metastases to assess cancer aggressiveness after systemic chemotherapy. Of 52 patients who received conversion therapy, high Ki-67 expression was detectable in 33 patients (63.5%) (Figure 2A), whereas low Ki-67 expression was detected in 19 patients (36.5%) (Figure 2B). The data indicate that a majority of the residual tumors contained high-grade cancer cells after effective chemotherapy (Supplemental Figure 1). High cell proliferative activity was observed in residual tumors with minimum sizes of 2 mm (Supplemental Figure 2). Interestingly, there was no significant association between the cell proliferative activity and the residual tumor size prior to conversion therapy (Table 2). High Ki-67 expression was significantly associated with high serum CEA level (Table 2). On the other hand, such the correlation between Ki-67 expression and serum CEA level was not detectable in straight hepatectomy group (Supplemental Table 1). Furthermore, we investigated the prognostic implications of the biological activity represented by Ki-67-positive cancer cells. High Ki-67 expression was significantly associated with a shorter disease-free survival compared to low Ki-67 expression (P < 0.001) (Figure 2C). The high Ki-67 expression group displayed more early disease recurrences within 1 year after conversion therapy (25 of 33 patients) than the low expression group (three of 19 patients) (76% vs. 16%). As for a recurrence pattern in high and low Ki-67 expression groups, an extra-hepatic recurrence was detectable in 36% (10 of 28 recurrent patients) and 43% (3 of 7 recurrent patients), respectively. Additionally, the high Ki-67 expression patients had significantly worse overall survival than the low Ki-67 expression patients (P < 0.01) (Figure 2C). On the other hand, as a control, we examined the Ki-67 expression of resected liver metastases also in 42 patients without systemic chemotherapy (straight hepatectomy group). Then, high Ki-67 expression was detectable in 28 patients (66.7%), whereas low Ki-67 expression was detected in 14 patients (33.3%). Interestingly, in contrast to conversion group, there was no significant difference in disease free survival and overall survival between low and high Ki-67 expressions in patients with straight hepatectomy (P = 0.14 and 0.74, respectively) (Figure 2D). By multivariate Cox regression analyses, high Ki-67 expression was significantly associated with shorter disease-free survival [hazard ratio [HR] 5.608, 95% confidence interval (CI) 2.484-13.615] and worse overall survival (HR 5.366, 95% CI 1.084-26.570) (Table 3). These results indicate that the Ki-67 marker can independently predict early recurrence and worse prognostic outcome due to the presence of residual micrometastases after conversion therapy. The other potential prognostic factors (tumor size, tumor number, CEA) did not predict early recurrence. Interestingly, even in the patients with RECIST PR (n = 32), high Ki-67 expression was significantly shorter disease-free survival (P < 0.001) (Supplemental Figure 3). These findings suggest that the residual high-grade proliferative activity represented by Ki-67-positive cells following systemic chemotherapy is a useful biomarker for worse prognostic outcomes after conversion therapy.
Figure 2.
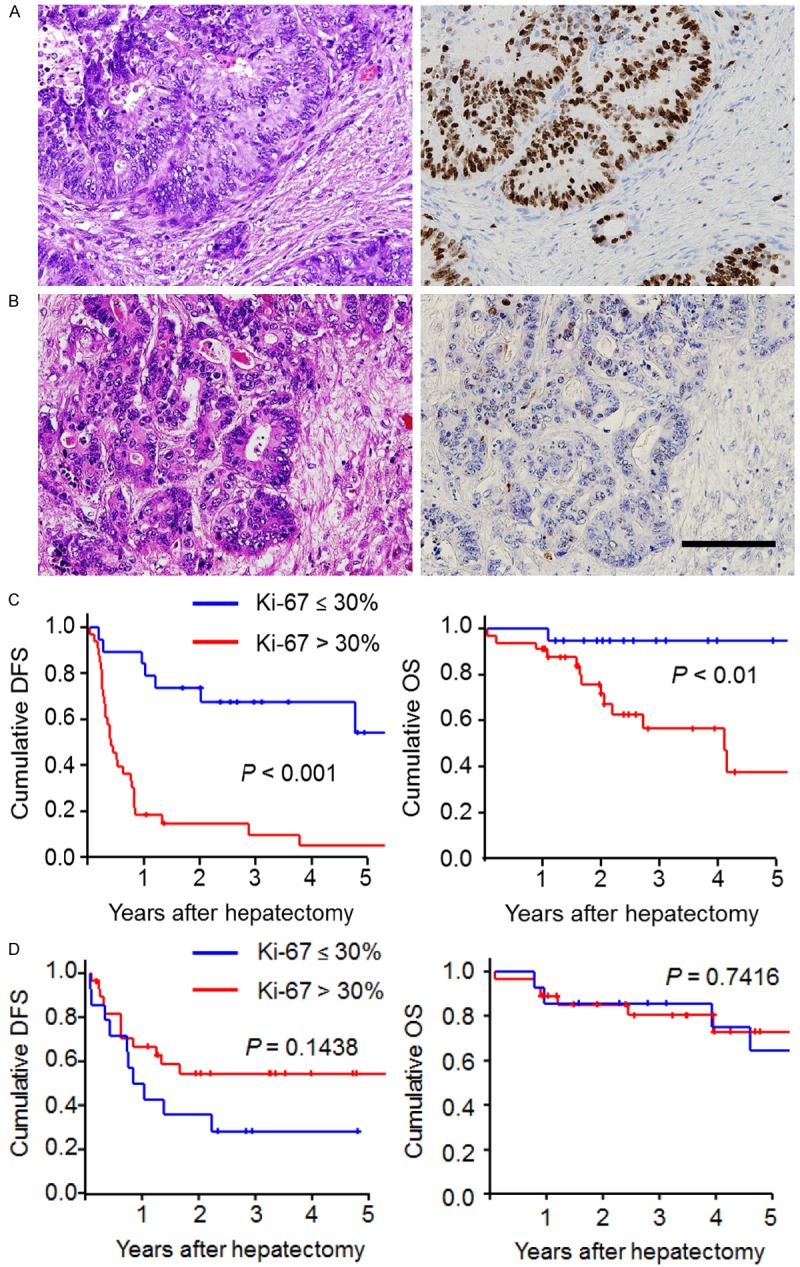
Immunohistochemical staining of Ki-67 expression in residual colorectal liver metastases after chemotherapy and its prognostic outcomes. A. Hematoxylin-eosin staining (left) and high Ki-67 expression (right); B. Hematoxylin-eosin staining (left) and low Ki-67 expression (right); (Scale bars, 100 µm); C. A Kaplan-Meier survival analysis of disease-free survival (left panel) and overall survival (right panel) between high and low Ki-67 expression levels in patients with conversion therapy; D. A Kaplan-Meier survival analysis of disease-free survival (left panel) and overall survival (right panel) between high and low Ki-67 expression levels in patients with straight hepatectomy. The log-rank test was used.
Table 2.
Comparison of clinical factors between low and high Ki-67 expression in patients with conversion therapy (n = 52)
| Variables | Low Ki-67 (n = 19) | High Ki-67 (n = 33) | P-value |
|---|---|---|---|
| Age | 69 (35-78) | 62 (45-83) | 0.065 |
| Sex (male/female) | 11 / 8 | 20/13 | 0.848 |
| Synchronous/Metachronous | 12/7 | 22/11 | 0.798 |
| Molecular targets (present/absent) | 7/12 | 19/14 | 0.150 |
| Tumor size prior to hepatectomy (mm) | 20 (3-160) | 31 (11-85) | 0.174 |
| Tumor number prior to hepatectomy | 2 (1-13) | 3 (1-19) | 0.126 |
| CEA prior to hepatectomy (ng/mL) | 4 (1.3-22.4) | 8 (1-173) | 0.004 |
| RECIST PR/SD or PD | 15/4 | 17/16 | 0.0502 |
Variable data was expressed with median (range). CEA, carcinoembryonic antigen; RECIST, response evaluation criteria in solid tumors (version 1.1).
Table 3.
Prognostic analyses of clinicopathological parameters in patients with conversion therapy for colorectal liver-limited metastases (n = 52)
| Parameters | HR | Exp (B) | P-value |
|---|---|---|---|
| Disease free survival | |||
| Univariate analysis | |||
| Tumor size > 3 cm | 1.882 | 0.975-3.632 | 0.060 |
| Tumor number ≥ 3 | 1.525 | 0.789-2.945 | 0.209 |
| CEA > 7 ng/mL | 2.795 | 1.388-5.631 | 0.004 |
| RECIST SD or PD | 1.674 | 0.849-3.302 | 0.137 |
| Ki-67 high | 5.608 | 2.382-13.204 | < 0.001 |
| Multivariate analysis | |||
| Ki-67 high | 5.608 | 2.382-13.204 | < 0.001 |
| Overall survival | |||
| Univariate analysis | |||
| Tumor size > 3 cm | 1.825 | 0.656-5.081 | 0.249 |
| Tumor number ≥ 3 | 1.471 | 0.546-3.964 | 0.446 |
| CEA > 7 ng/mL | 2.521 | 0.874-7.275 | 0.087 |
| RECIST SD or PD | 4.355 | 1.569-12.087 | 0.005 |
| Ki-67 high | 6.081 | 1.324-27.934 | 0.02 |
| Multivariate analysis | |||
| RECIST SD or PD | 4.299 | 1.402-12.755 | 0.01 |
| Ki-67 high | 5.366 | 1.084-26.570 | 0.04 |
Cox regression analyses were used. CEA, carcinoembryonic antigen; RECIST, response evaluation criteria in solid tumors (version 1.1).
Discussion
In this study, conversion therapy provided significantly improved prognostic outcomes compared to patients with unresectable liver metastases in previous studies [26,27]. One remaining concern is the high recurrence rate after conversion therapy. Ki-67 antigen is expressed during all active phases of the cell cycle (G1, S, G2, and mitosis) and has become a widely used to determine the proliferative potential of a tumor [28]. Although the expression level of Ki-67 in the colorectal liver metastases after chemotherapy has been unknown until now, a majority (63.5%) of the residual tumors even after effective chemotherapy contained biologically active cancer cells with high Ki-67 expression levels in the present study. Surprisingly, high-grade activity was detected in residual tumors with minimum sizes of 2 mm, and there was no significant association between the cell proliferative activity and the residual tumor size in the presents study. Previously, elevated CEA level after preoperative chemotherapy was associated with the shorter disease-free survival after hepatectomy in patients with colorectal liver metastases [29]. In the present study, high Ki-67 expression was significantly associated with elevated CEA level prior to hepatectomy, whereas such the correlation was not detectable in straight hepatectomy group. Furthermore, we identified high Ki-67 expression as a useful biomarker for high risk of early recurrence and death from disease, even after conversion therapy. The high Ki-67 expression group displayed remarkably early recurrence within 1 year after conversion therapy compared to the low expression group (76% vs. 16% at 1 year after conversion therapy). These findings suggest that the high Ki-67 expression in the resected specimens is a biomarker of residual micrometastases containing aggressive cancer cells after conversion therapy. The use of adjuvant chemotherapy may be required to abolish any residual micrometastases in the remnant liver. This is the first study that provides the expression level of Ki-67 in the colorectal liver metastases after chemotherapy and its prognostic impact after conversion therapy.
In contrast to the clinical impact of Ki-67 expression on prognostic outcomes after conversion therapy (conversion group), Ki67 expression level in colorectal liver metastases without systemic chemotherapy (straight hepatectomy group) did not show any association with prognostic outcomes after hepatectomy in the present study nevertheless the similar ratio of high Ki-67 expression (63.5% in conversion and 66.7% in straight hepatectomy). Jong et al. [30] examined the Ki-67 expression level in 45 liver metastases without chemotherapy and reported that low (less than 33%) and high (33% to 100%) expression levels of Ki-67 were detectable in 35% and 65%, respectively. Their Ki-67 expression level in colorectal liver metastases without chemotherapy was similar to our results. Previous studies have reported conflicting results regarding the association between Ki-67 expression status and prognosis in colorectal liver metastases without chemotherapy (straight hepatectomy group) [30-32]. While Petrowsky et al. [31] reported that high Ki-67 expression had significantly shorter median survival compared with those with low scores and a high Ki-67 expression was an independent negative prognostic factor by multivariate regression analysis in 41 patients with colorectal liver metastases, Anjomshoaa et al. [32] reported that slow proliferation represented by Ki-67 immunostaining was a biological characteristic of liver metastases with the ability to metastasize in 27 liver metastasis. In the present study, Ki-67 expression level in straight hepatectomy group is not associated with prognostic outcomes after hepatectomy, and this is in agreement with another study [30]. Thus, a clinical relevance of ki-67 expression in straight hepatectomy group is still controversial. The clinical value of Ki-67 expression in relation to prognosis in colorectal liver metastases without preoperative chemotherapy cannot yet be applied in the clinical situation and should be further investigated.
The first limitation was that this study was retrospective study and its small sample size. Second limitation was a validity of cut-off value in Ki-67 expression. Although a clear cut-off value for Ki-67 in liver metastases after chemotherapy has not been established, we could successfully distinguish the patients with better and worse prognoses after conversion therapy, but not after straight hepatectomy, using a cut-off value of 30%. A large and prospective study is required to re-evaluate the validity of Ki-67 as a prognostic factor and its cut-off value in future.
In conclusion, Ki-67 expression is a useful biomarker for patients with high potential risk of early recurrence and death from disease after conversion therapy for initially unresectable liver metastases. The high Ki-67 expression level in the resected specimens may be a biomarker of residual micrometastases containing aggressive cancer cells, even after effective chemotherapy.
Acknowledgements
This work was supported by Grant-in-Aid for Young Scientists (B); the Ministry of Education, Culture, Sports, Science, and Technology of Japan, No. 24791434 (to H.H.); and Japanese Foundation for Multidisciplinary Treatment of Cancer, Japan (to H.H.).
Disclosure of conflict of interest
There is no conflicts of interest or financial ties to disclose.
Abbreviations
- CRC
colorectal cancer
- S-1
an oral fluoropyrimidine
- CEA
carcinoembryonic antigen
Supporting Information
References
- 1.Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer. 2002;38:1023–1033. doi: 10.1016/s0959-8049(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 2.Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, Vellone M, Giuliante F, Nuzzo G, Barone C. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 3.Kohne CH, van Cutsem E, Wils J, Bokemeyer C, El-Serafi M, Lutz MP, Lorenz M, Reichardt P, Ruckle-Lanz H, Frickhofen N, Fuchs R, Mergenthaler HG, Langenbuch T, Vanhoefer U, Rougier P, Voigtmann R, Muller L, Genicot B, Anak O, Nordlinger B. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J. Clin. Oncol. 2005;23:4856–4865. doi: 10.1200/JCO.2005.05.546. [DOI] [PubMed] [Google Scholar]
- 4.Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, Levitt R, Rowland K, Nair S, Sargent DJ, Donohue JH. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J. Clin. Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 5.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, Konopke R, Stroszczynski C, Liersch T, Ockert D, Herrmann T, Goekkurt E, Parisi F, Kohne CH. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 6.Beppu T, Miyamoto Y, Sakamoto Y, Imai K, Nitta H, Hayashi H, Chikamoto A, Watanabe M, Ishiko T, Baba H. Chemotherapy and Targeted Therapy for Patients with Initially Unresectable Colorectal Liver Metastases, Focusing on Conversion Hepatectomy and Long-Term Survival. Ann Surg Oncol. 2014;21(Suppl 3):S405–13. doi: 10.1245/s10434-014-3577-x. [DOI] [PubMed] [Google Scholar]
- 7.Primrose JN. Surgery for colorectal liver metastases. Br J Cancer. 2010;102:1313–1318. doi: 10.1038/sj.bjc.6605659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lise M, Mocellin S, Pilati P, Nitti D. Colorectal liver metastasis: towards the integration of conventional and molecularly targeted therapeutic approaches. Front Biosci. 2005;10:3042–3057. doi: 10.2741/1761. [DOI] [PubMed] [Google Scholar]
- 9.Okines A, Puerto OD, Cunningham D, Chau I, Van Cutsem E, Saltz L, Cassidy J. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer. 2009;101:1033–1038. doi: 10.1038/sj.bjc.6605259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohne CH, Lenz HJ. Chemotherapy with targeted agents for the treatment of metastatic colorectal cancer. Oncologist. 2009;14:478–488. doi: 10.1634/theoncologist.2008-0202. [DOI] [PubMed] [Google Scholar]
- 11.Masi G, Loupakis F, Pollina L, Vasile E, Cupini S, Ricci S, Brunetti IM, Ferraldeschi R, Naso G, Filipponi F, Pietrabissa A, Goletti O, Baldi G, Fornaro L, Andreuccetti M, Falcone A. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249:420–425. doi: 10.1097/SLA.0b013e31819a0486. [DOI] [PubMed] [Google Scholar]
- 12.Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, Sobrero A, Ychou M. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985–992. doi: 10.1093/annonc/mdn735. [DOI] [PubMed] [Google Scholar]
- 13.Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, Nitti D, Torri V, Elias D, O’Callaghan C, Langer B, Martignoni G, Bouche O, Lazorthes F, Van Cutsem E, Bedenne L, Moore MJ, Rougier P. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J. Clin. Oncol. 2008;26:4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 14.Ychou M, Hohenberger W, Thezenas S, Navarro M, Maurel J, Bokemeyer C, Shacham-Shmueli E, Rivera F, Kwok-Keung Choi C, Santoro A. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol. 2009;20:1964–1970. doi: 10.1093/annonc/mdp236. [DOI] [PubMed] [Google Scholar]
- 15.Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B, Bugat R, Lazorthes F, Bedenne L. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J. Clin. Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 16.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 17.Kelley RK, Van Bebber SL, Phillips KA, Venook AP. Personalized medicine and oncology practice guidelines: a case study of contemporary biomarkers in colorectal cancer. J Natl Compr Canc Netw. 2011;9:13–25. doi: 10.6004/jnccn.2011.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mocellin S, Lise M, Nitti D. Targeted therapy for colorectal cancer: mapping the way. Trends Mol Med. 2005;11:327–335. doi: 10.1016/j.molmed.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478–483. doi: 10.1053/hupa.2002.124123. [DOI] [PubMed] [Google Scholar]
- 20.van Rhijn BW, Vis AN, van der Kwast TH, Kirkels WJ, Radvanyi F, Ooms EC, Chopin DK, Boeve ER, Jobsis AC, Zwarthoff EC. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J. Clin. Oncol. 2003;21:1912–1921. doi: 10.1200/JCO.2003.05.073. [DOI] [PubMed] [Google Scholar]
- 21.Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, Pusztai L, Symmans WF, Kuerer HM, Mittendorf EA, Hortobagyi GN, Meric-Bernstam F. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010;28:1821–1828. doi: 10.1200/JCO.2009.25.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvesen HB, Iversen OE, Akslen LA. Prognostic significance of angiogenesis and Ki-67, p53, and p21 expression: a population-based endometrial carcinoma study. J. Clin. Oncol. 1999;17:1382–1390. doi: 10.1200/JCO.1999.17.5.1382. [DOI] [PubMed] [Google Scholar]
- 23.Mima K, Beppu T, Chikamoto A, Miyamoto Y, Nakagawa S, Kuroki H, Okabe H, Hayashi H, Sakamoto Y, Watanabe M, Kikuchi K, Baba H. Hepatic resection combined with radiofrequency ablation for initially unresectable colorectal liver metastases after effective chemotherapy is a safe procedure with a low incidence of local recurrence. Int J Clin Oncol. 2013;18:847–855. doi: 10.1007/s10147-012-0471-z. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi H, Sakai K, Baba H, Sakai T. Thrombospondin-1 is a novel negative regulator of liver regeneration after partial hepatectomy through transforming growth factor-beta1 activation in mice. Hepatology. 2012;55:1562–1573. doi: 10.1002/hep.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi H, Beppu T, Okabe K, Masuda T, Okabe H, Baba H. Risk factors for complications after partial splenic embolization for liver cirrhosis. Br J Surg. 2008;95:744–750. doi: 10.1002/bjs.6081. [DOI] [PubMed] [Google Scholar]
- 26.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghemard O, Levi F, Bismuth H. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adam R, Wicherts DA, de Haas RJ, Ciacio O, Levi F, Paule B, Ducreux M, Azoulay D, Bismuth H, Castaing D. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J. Clin. Oncol. 2009;27:1829–1835. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 28.Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- 29.Yi JH, Kim H, Jung M, Shin SJ, Choi JS, Choi GH, Baik SH, Min BS, Kim NK, Ahn JB. Prognostic factors for disease-free survival after preoperative chemotherapy followed by curative resection in patients with colorectal cancer harboring hepatic metastasis: a single-institute, retrospective analysis in Asia. Oncology. 2013;85:283–289. doi: 10.1159/000355475. [DOI] [PubMed] [Google Scholar]
- 30.De Jong KP, Stellema R, Karrenbeld A, Koudstaal J, Gouw AS, Sluiter WJ, Peeters PM, Slooff MJ, De Vries EG. Clinical relevance of transforming growth factor alpha, epidermal growth factor receptor, p53, and Ki67 in colorectal liver metastases and corresponding primary tumors. Hepatology. 1998;28:971–979. doi: 10.1002/hep.510280411. [DOI] [PubMed] [Google Scholar]
- 31.Petrowsky H, Sturm I, Graubitz O, Kooby DA, Staib-Sebler E, Gog C, Kohne CH, Hillebrand T, Daniel PT, Fong Y, Lorenz M. Relevance of Ki-67 antigen expression and K-ras mutation in colorectal liver metastases. Eur J Surg Oncol. 2001;27:80–87. doi: 10.1053/ejso.2000.1029. [DOI] [PubMed] [Google Scholar]
- 32.Anjomshoaa A, Nasri S, Humar B, McCall JL, Chatterjee A, Yoon HS, McNoe L, Black MA, Reeve AE. Slow proliferation as a biological feature of colorectal cancer metastasis. Br J Cancer. 2009;101:822–828. doi: 10.1038/sj.bjc.6605229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


