Fig. 1.
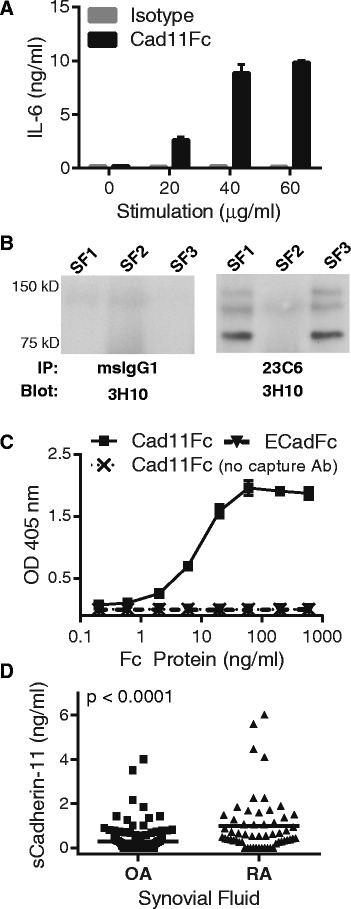
Evidence for cadherin-11 extracellular domain shedding in osteoarthritis (OA) and rheumatoid arthritis (RA) synovium. (a) Serum-starved RA synovial fibroblasts were incubated overnight with increasing concentrations of cad11Fc or isotype control antibody. IL-6 release was then measured by ELISA. Similar results were previously published and further characterized as discussed [3]. (b) Synovial fluid samples from OA patients were immunoprecipitated with a cadherin-11 extracellular domain antibody (23C6) followed by western blot analysis with a distinct cadherin-11 antibody (3H10). (c) Detection of the chimeric proteins cadherin-11-Fc or E-cadherin-Fc was measured using an ELISA developed to recognize the human cadherin-11 extracellular domain. The ELISA capture antibody was removed from the assay to serve as a negative control. The ELISA uses two anti-cadherin-11 monoclonal antibodies (23C6 and 3H10) recognizing distinct extracellular epitopes. (d) Soluble cadherin-11 fragments were detected by cadherin-11 extracellular domain specific ELISA in OA (n = 143, mean +/− standard deviation 0.28 +/− 0.56 ng/ml) and RA (n = 57, mean +/− standard deviation 0.99 +/− 1.3 ng/ml) synovial fluid patient samples. Cadherin-11 levels were significantly higher in RA synovial fluid (P <0.0001, two-tailed Student t-test)
